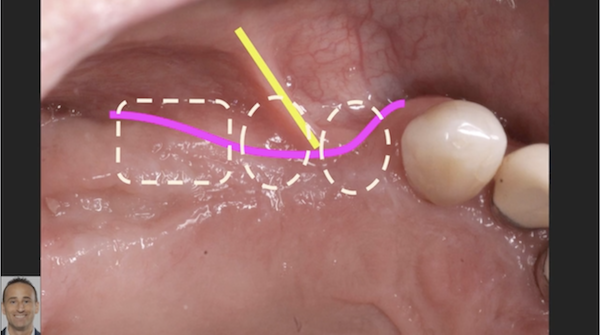
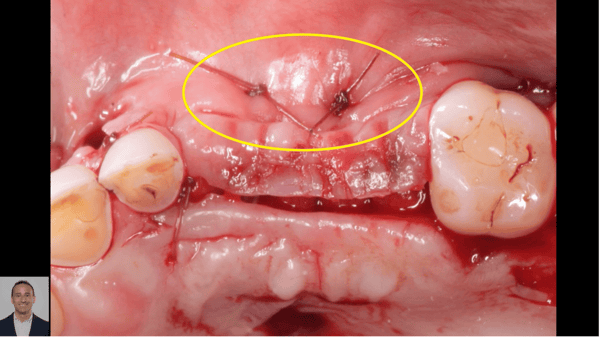
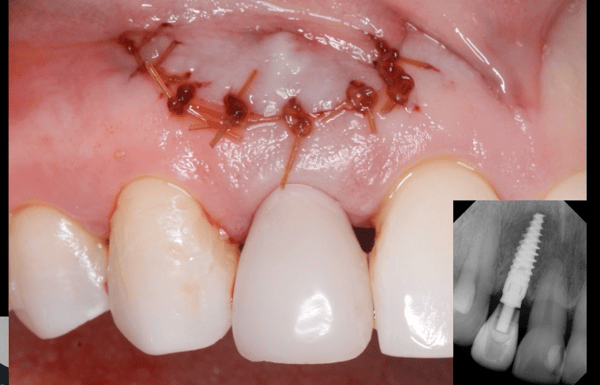
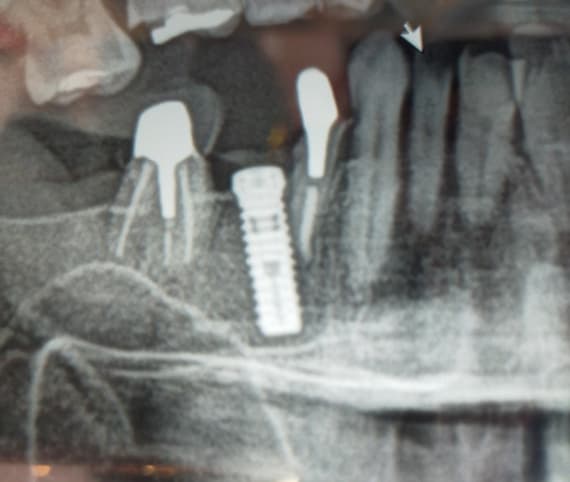
Measuring Osseointegration
Osseonews (ON): Dr. Stanford could you describe the current major study that Astra Tech has launched to collect and analyze clinical performance data on their implant system?
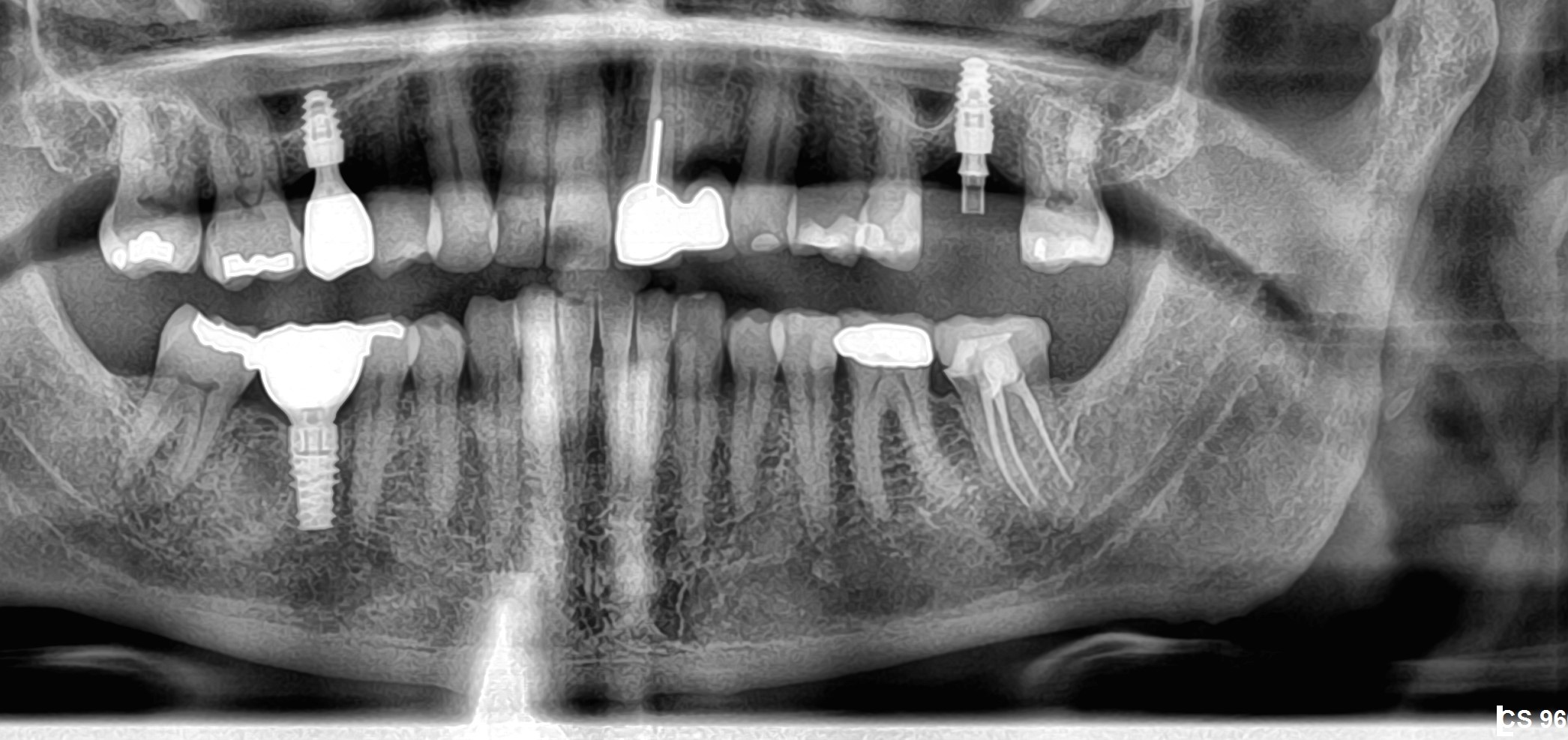
Dr. Stanford: First, I need to say that what I am discussing are studies that are ongoing. The studies are not complete, and the data have not been reviewed by the US Food and Drug Administration. With that in mind, it is exciting to report that Astra Tech is involved in collecting data in two major studies concerning implant longevity in the partially edentulous posterior mandible and posterior maxilla.
The mandibular posterior implant sites had 79% Type I and II bone. Implants were placed in the partially edentulous mandible and provisional restorations were delivered the same day as placement. After 6 weeks, the permanent fixed partial dentures were delivered. After 1 year post-loading, the rate of success is still at 100%.
Results for the maxillary posterior are also excellent. This is remarkable because of the general poor bone quality and generally poorer prognosis of implants in this site. The maxillary posterior implant sites had 54% Type IV bone and 46% Type III bone. Implants were placed and allowed to integrate for a period of six weeks. Implant lengths ranged from 8-13mm. Provisional restorations were then placed at 6 weeks.
A small number of implants were mobile at 6 weeks and additional healing time was allowed resulting in increased stability that allowed the majority to be provisionally restored after an additional 6 weeks of healing. Permanent fixed partial dentures were placed at 1 year. Implant failures occurred prior to loading. After one year of loading the success rate is 97% for all implants placed.
We will continue to monitor both sets of patients for a full five years. There are three different test sites for each study with two in the United States and one in Europe. We are very pleased with the high success rate.
ON: How did you measure the osseointegration and bone quality?
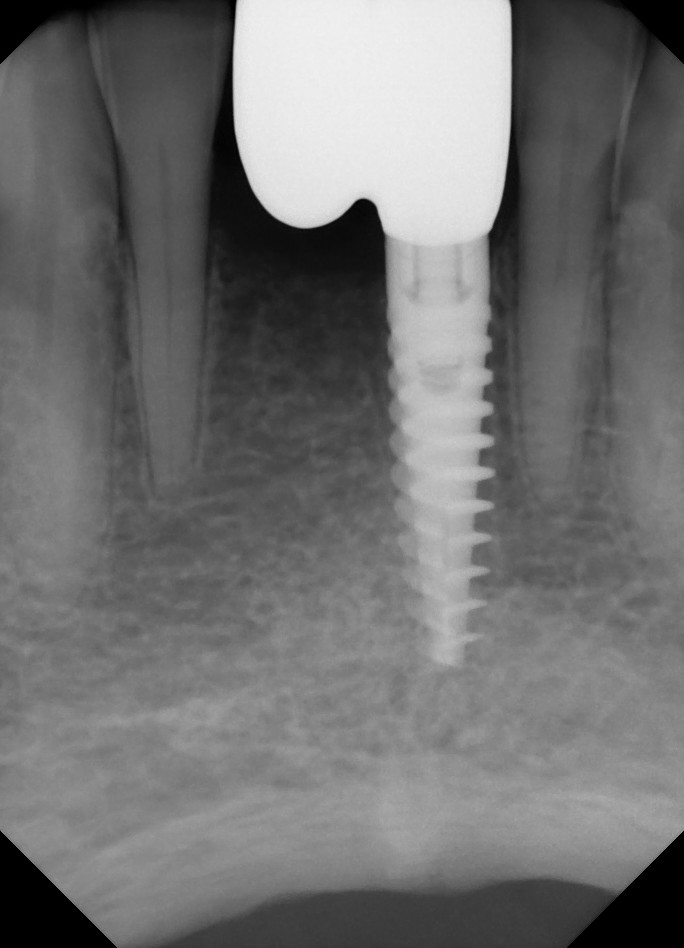
Dr. Stanford: We used resonance frequency analysis. This is a non-invasive technique for assessing implant stability. The device generates harmonic sound waves which are directed to the implant and surrounding bone and a transducer measures the dampening of the waves. This provides a quantitative assessment. The scale of measurement is the Implant Stability Quotient (ISQ) and the Osstell Device (Integration Diagnostics, Savedalen, Sweden). Increases in ISQ measurements are a measure of improved bone stiffness and thus healing around the implant.
ON: This technique then provides parametric data?
Dr. Stanford: That is correct. The data provides a longitudinal assessment of quality of the surrounding bone, bone to implant contact and the relative stiffness of the implant system. The value is the ability to follow, in time, the healing process of each implant as a measure of the changing stiffness of the bone.
ON: In these studies you were using implants with the fluoride-modified surface?
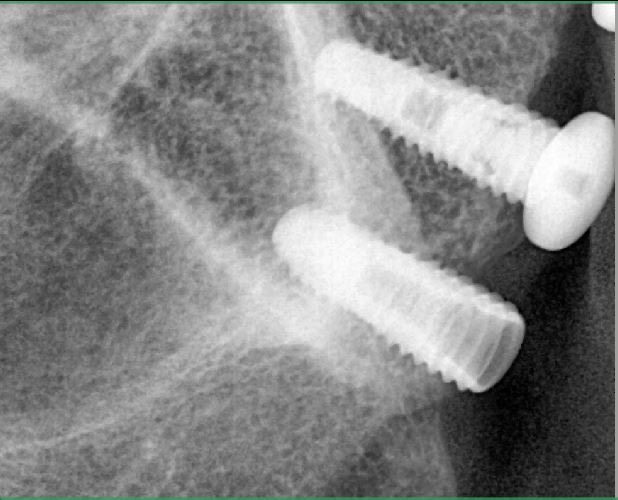
Dr. Stanford: That is correct. The implant surfaces are blasted with titanium dioxide and subjected to a dilute hydrofluoric acid solution at a constant pH and temperature. Fluoride ions bind to the titanium forming fluoroxides. The fluoride constituent stimulates bone growth along the implant-bone interface, so you have bone matrix growing from the walls of the implant as well as bone growing from the lateral walls of the osteotomy. This is similar to the use of titanium hexafluoride to stimulate the remineralization of enamel.
The end result is rapid bone growth around the implant producing more rapid osseointegration. From a clinical perspective this could lead to earlier loading and/or improved implant success in poor quality bone. This is especially important in the posterior maxilla where the bone quality is the poorest and the chance of success the lowest.
Astra Tech has named this surface OsseoSpeedâ„¢.
ON: Can you describe the other major international study that you are directing?
Dr. Stanford: This study is also sponsored by Astra Tech and I am the chair of the independent steering committee. The study is called Fixture Osseospeed Clinical Outcome Systemic Evaluation (FOCUS). To date, we have recruited 124 dental clinics in 14 countries to field test the OsseoSpeedâ„¢ Implant. The goal is to assess the indications that dentists chose to place this implant, the implant success, along with measurement of bone support and esthetic success for partially edentulous situations.
ON: So what you really wanted here was a true, wide scale, clinical field test with many practitioners placing and restoring the implants in real clinical situations other than the rigidly controlled setting of a university?
Dr. Stanford: In this way we felt that we would be collecting data that accurately reflected clinical reality in real-world dentistry.
Over 2400 implants have been placed in 624 patients with over 1500 having already been restored. We are now out over 2 years and the survival rate is running over 99%. We will continue to collect and analyze the data for at least 5 years after implant placement. The results are very encouraging.
ON: Astra Tech is sponsoring a World Congress in New York City in April 2006?
Dr. Stanford: Yes. The Congress will run from April 6-8, 2006 at the New York Hilton in New York City. The key focus of the Astra Tech World Congress is to bring together dental professionals from all over the world. This international congress and lectures will be simultaneously translated and broadcasted in Spanish, French, Italian and German. Astra Tech will also support the advance of the scientific study of implants, and to that end, will be presenting a $10,000 award to an individual or group for making progress in research in osseointegration.
There will be a great deal of information presented by world-class speakers on implant dentistry and osseointegration. Courses and lectures will include: Prosthetic hands-on presentations, surgery, site development, osseointegration, abutment design, esthetics, techniques for soft tissue management, laboratory technicians courses and Practice Management. There will be specific workshops for dentists, assistants and laboratory technicians, as well.
Interviewed provided by Dr. Gary Kaplowitz, Special Editor for OsseoNews.com