Failed Nobel Tapered Groovy Implant #30: Possible Causes?
I was wondering if anyone may be able to provide insight into why this case failed on me.
Background:
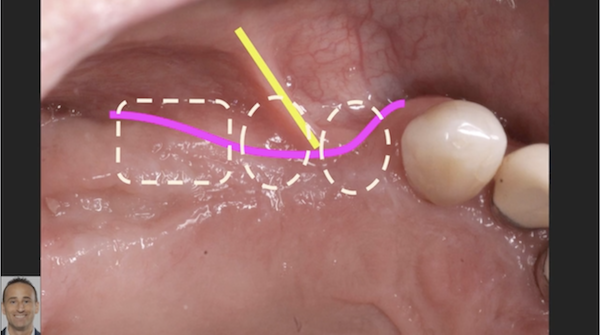
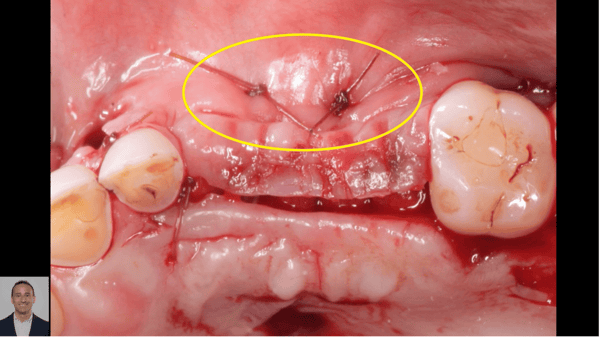
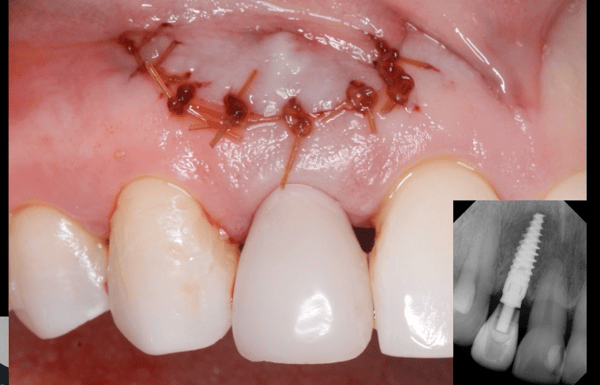
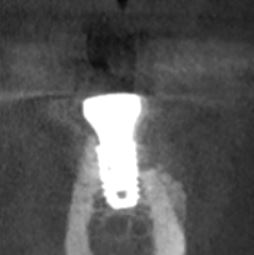
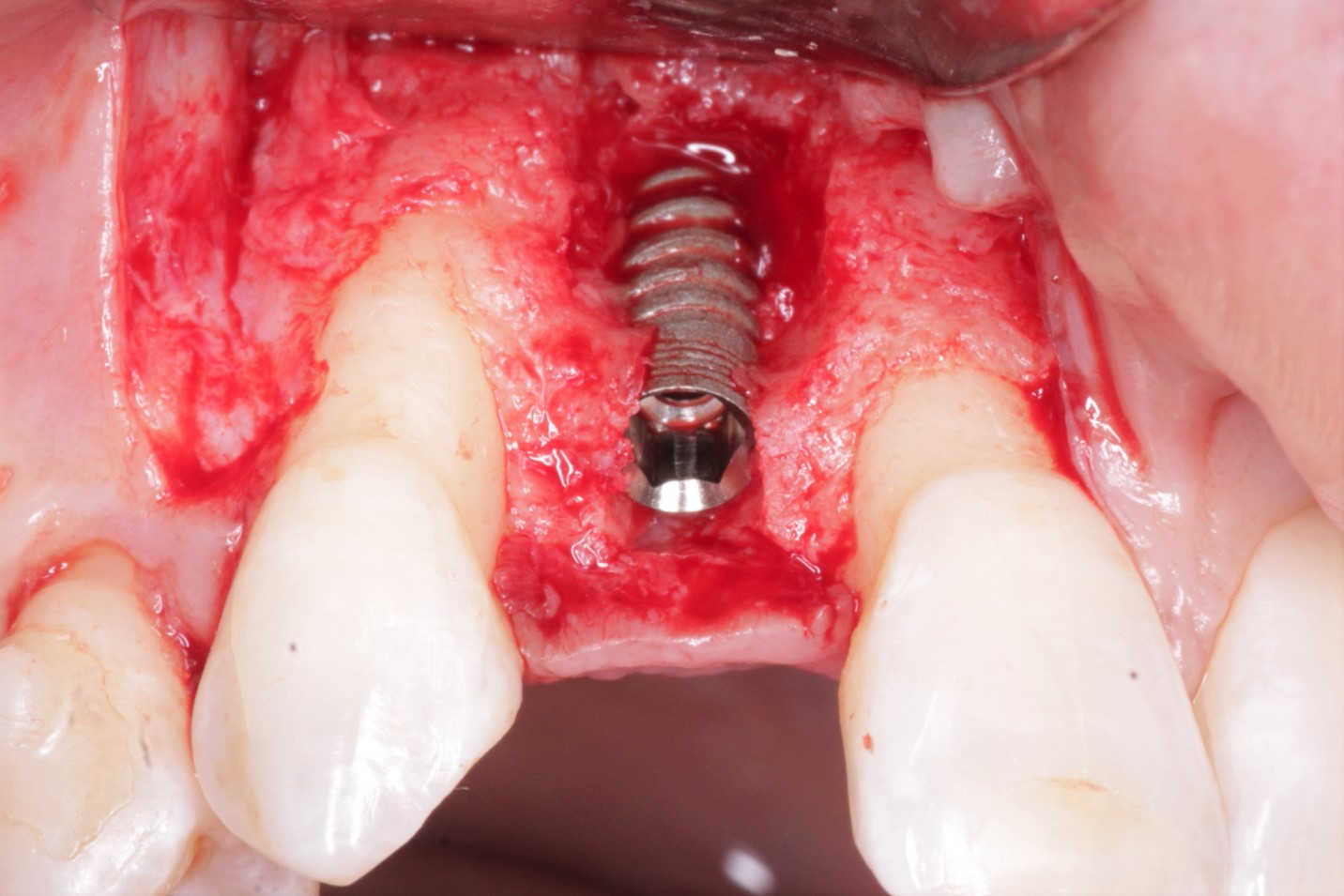
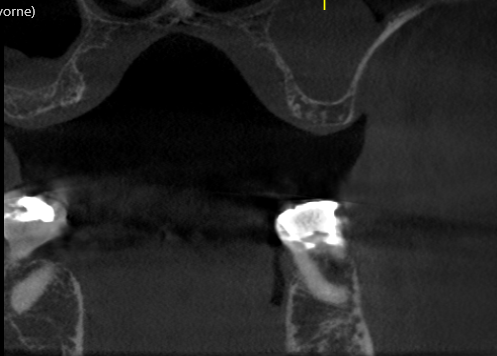
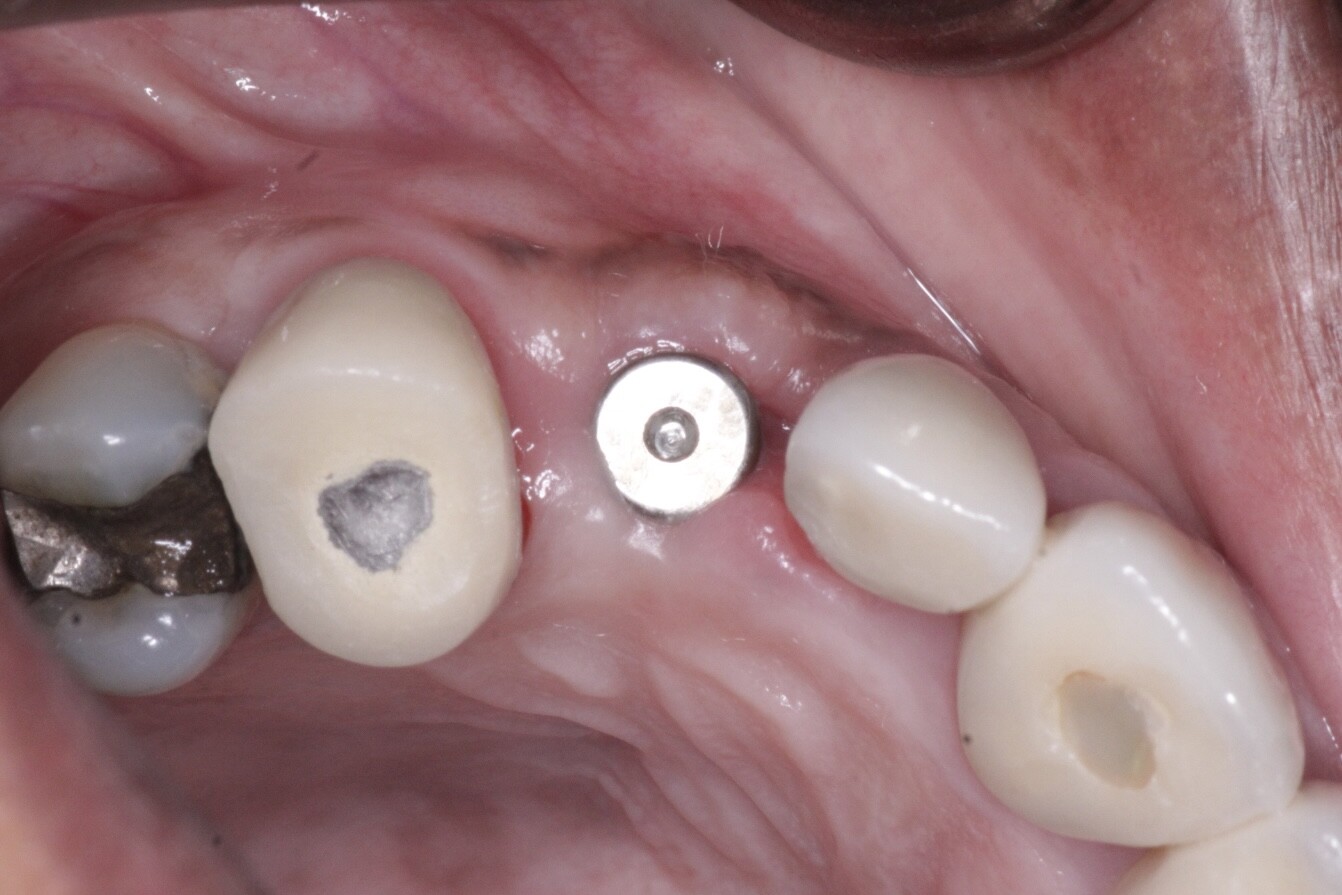
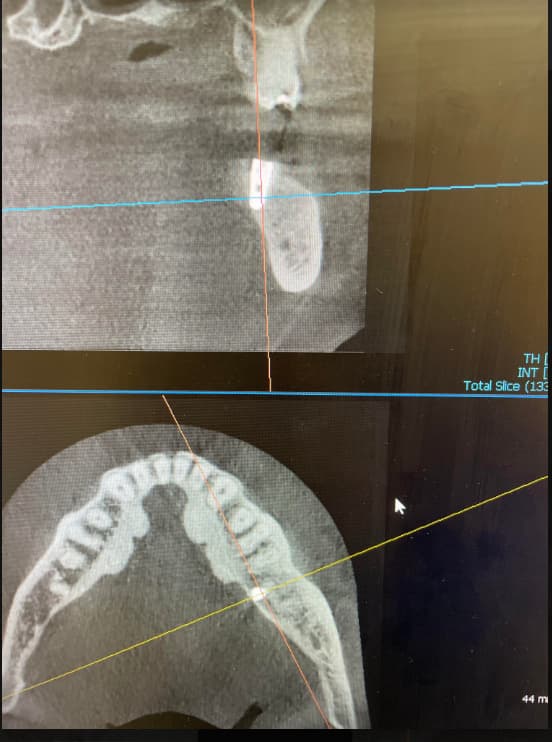
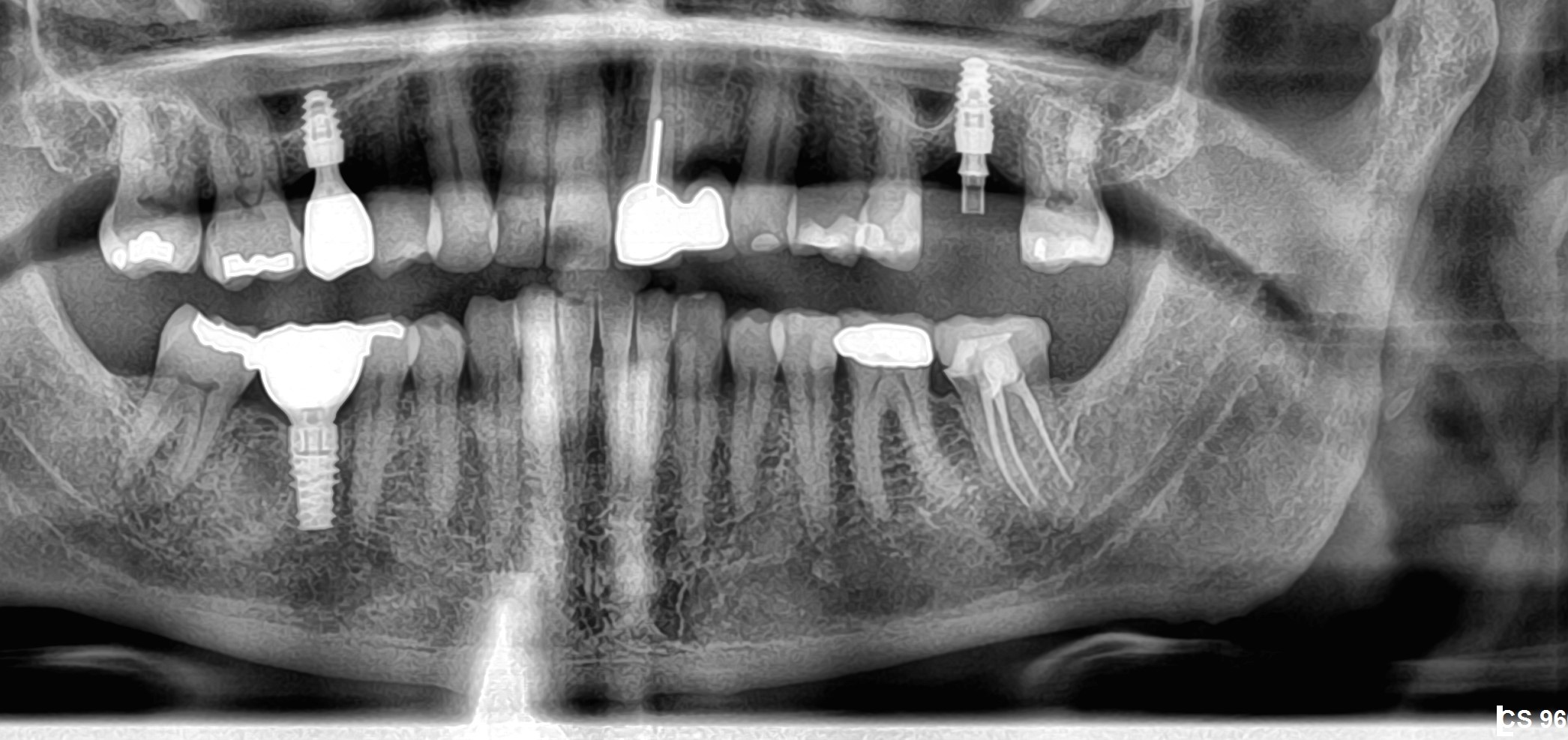
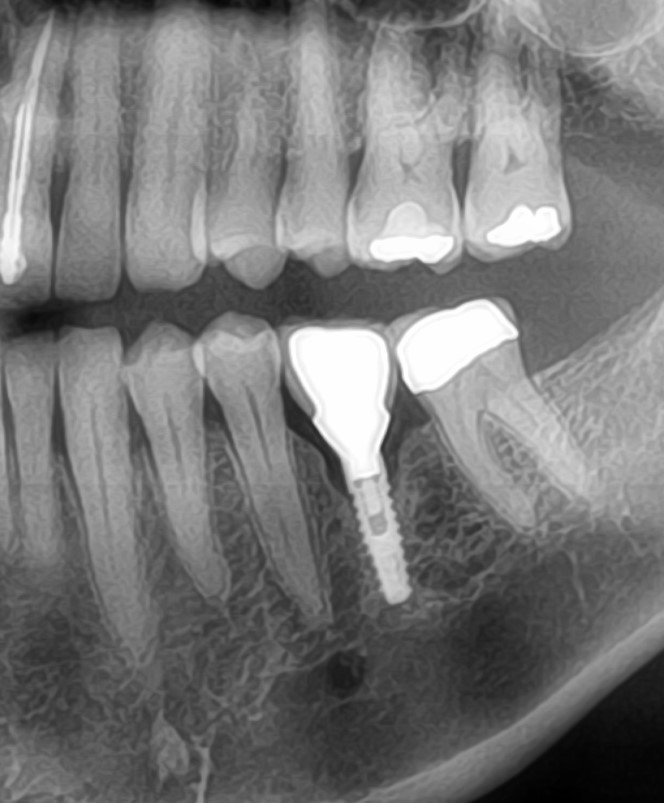
Patient presented as an emergency with failed a root canal on #30 (mandibular right first molar site). After discussing treatment options, she wanted to proceed with an implant. We extracted, grafted with Osteokor Cortico-Cencellous. She followed up for comprehensive care and maintained hygiene recalls. Finances limited placing an implant and we had to hold of for 12 months. After 12 months, we placed a Nobel Tapered Groovy 5×13 with torque of 35+ Ncm following recommended protocol with the addition of betadine irrigation at final stage for 1 minute. Pt returned 10 days for post op and suture removal, complaining of slight discomfort controlled with ibuprofen. Slight pressure placed on the healing abutment with the driver, rotated the implant and continue to unscrew the implant out of the site. We have regrafted the site and are monitoring healing. Med history is unremarkable.
Questions:
Any comments on probable causes for failure? I have used the same protocol for all of my cases and this is a first failure I had. The only thing that I keep coming back to is that perhaps I overheated the bone when placing the implant. When can I attempt another implant installation?
Case Images: