FDA Clears New Collagen Biomaterials
Osseous Technologies of America has announced that they have received FDA clearance on their new line of patented Collagen Biomaterials. OTA’s line of Collagen products will be available in a variety of configurations for virtually every application related to
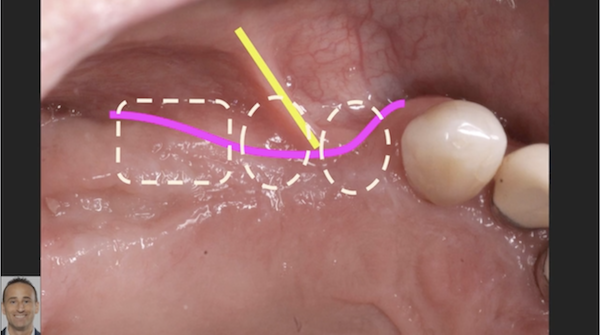
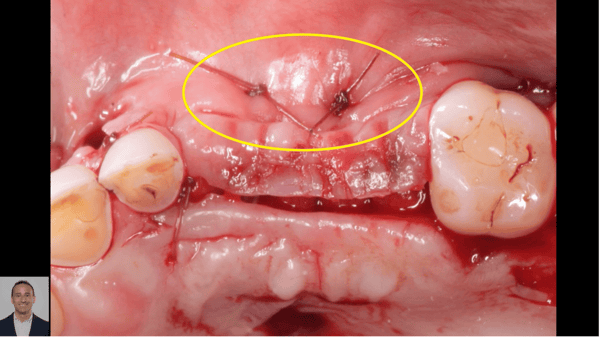
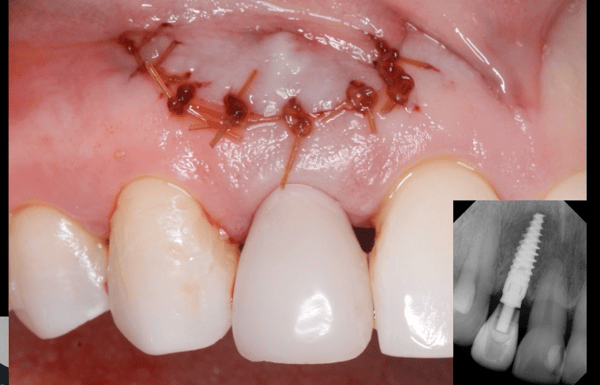
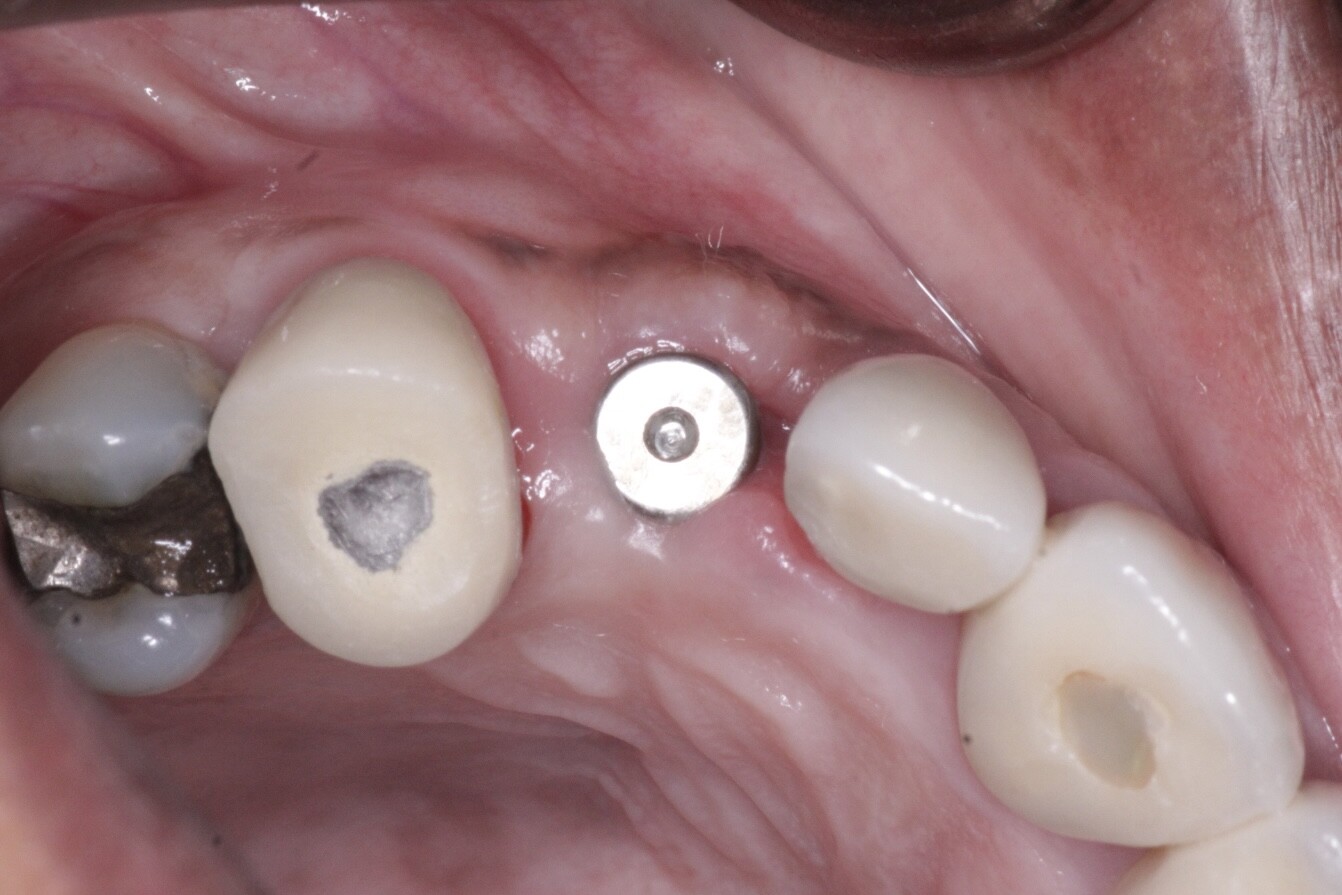
pre-prosthetic and bone reconstruction surgery. It provides containment of any type of graft material so that it will not migrate from the placement site.
The Socket Capsule, Sinus Capsule, BioSphere and Wedge are intended to be a solution for success for anyone placing dental implants. OTA Collagen Biomaterial is resorbable and intended for placement in the areas of dental implants, bone defects or ridge reconstruction to aid in wound healing post dental surgery.
Founded in 2003, Osseous Technologies of America began its current operations to participate in the rapidly expanding dental market for hard and soft tissue products in dental implant surgery. OTA is the exclusive vendor of the Balloon for Sinus Lifts and offers a wide range of products and services with the convenience and advantages of an “off the shelf productâ€.
#########
For more information:
Janet L. May, Operations Manager (949)250-0184, janet@osseoustech.com
(mailto:janet@osseoustech.com)