Fosamax Drug Could Be Merck’s Next Woe
Even the Wall Street Journal is beginning to comment on the Fosamax debate. This past week the newspaper commented: "Echoing claims made in Vioxx lawsuits, Pensacola, Fla.-based law firm
Levin Papantonio Thomas Mitchell Echsner & Proctor earlier this
week filed a suit against Merck in U.S. District Court in Fort Myers
seeking class-action status. The suit claims that Merck sold and
heavily marketed Fosamax, the company’s second-best-selling product, as
safe despite knowing about its dangerous side effect. Another law firm,
Knoxville, Tenn.-based Threadgill, filed a similar suit against Merck
last fall."
With Fosamax, attorneys are closely following the Vioxx playbook while claiming that they have more powerful cases because the link between Fosamax and the disease is easier to establish.
"With Vioxx, Merck can get away with saying, ‘Hey, a lot of people have heart attacks for a number of reasons that have nothing to do with our product," says Tim O’Brien, a lawyer with Levin Papantonio. "But this is a very unique condition that isn’t caused by cigarette-smoking or eating cheeseburgers."
Merck says most of the reported cases of osteonecrosis in patients taking bisphosphonates — the class of drugs Fosamax belongs to — have been cancer patients injected with more-powerful intravenous forms, and that a direct link to Fosamax isn’t so easily drawn.
"The cause of osteonecrosis of the jaw (ONJ) is not well understood and is likely to include a number of conditions," the company says in a statement.
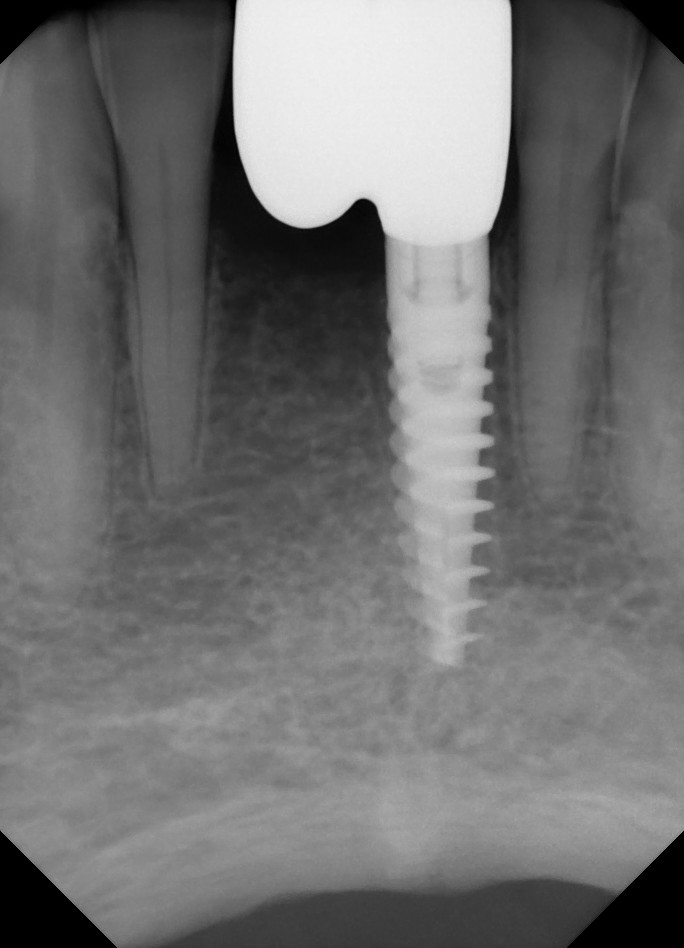
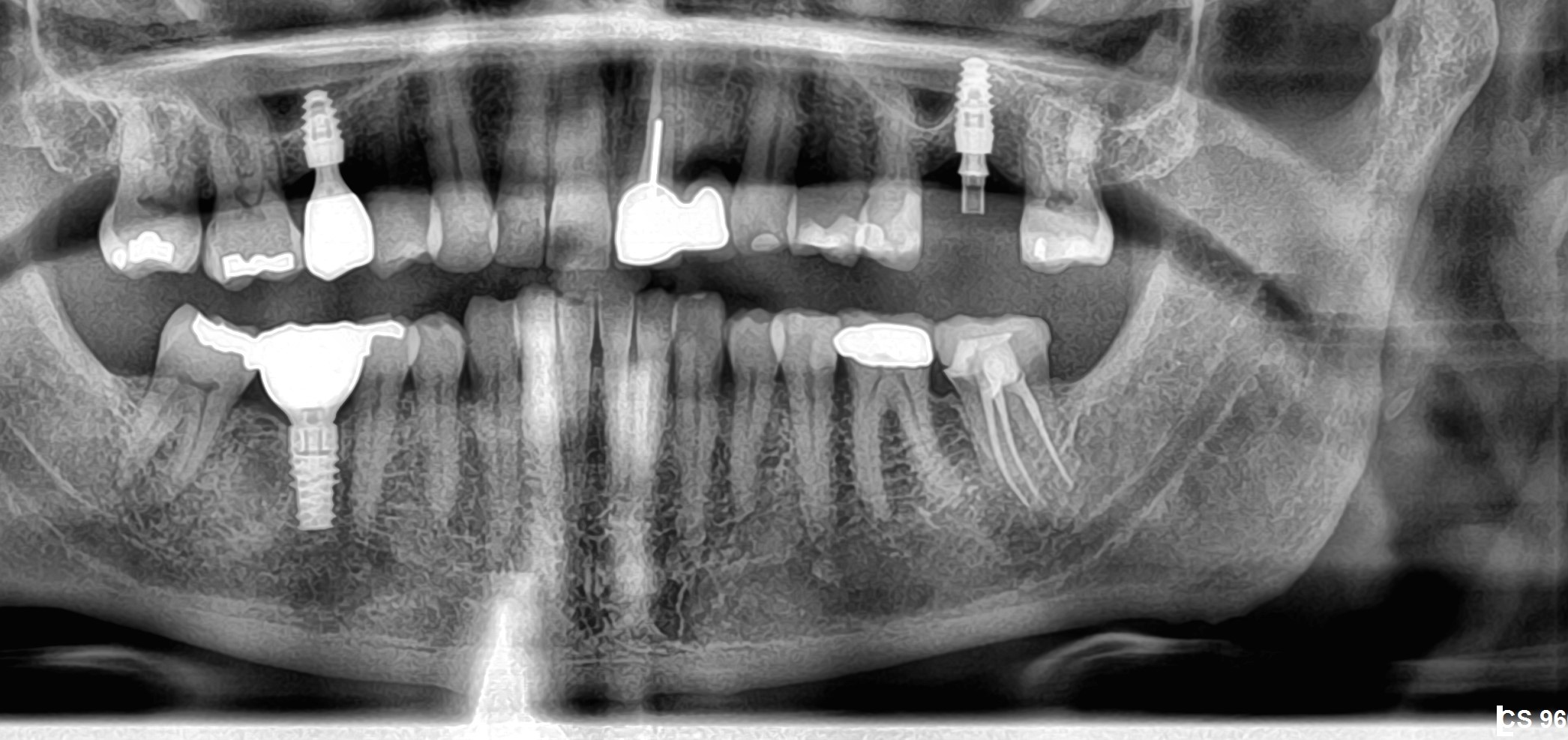
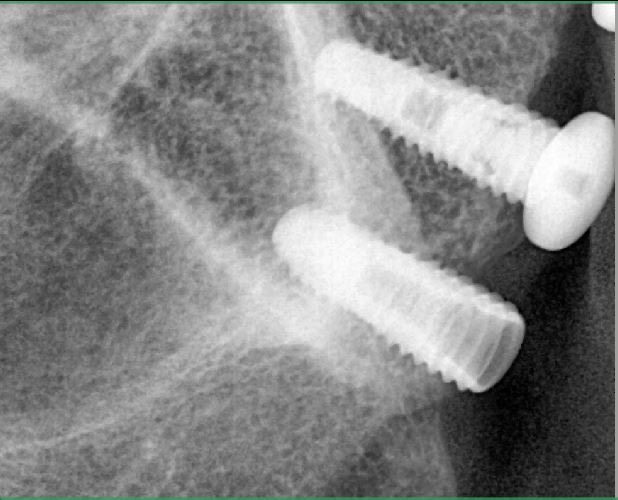
Oral surgeons and dentists began noticing the link between jaw decay and bisphosphonates five years ago. At first they thought that only the potent, intravenous versions of the drugs, such as those administered to cancer patients to stop cancer cells from dissolving bone, posed a risk.
In the past two years, some oral surgeons have become convinced that oral bisphosphonates such as Fosamax can also cause jawbone death when taken for a long period of time. Last month, the American Association of Endodontists issued a position statement recommending that dental surgeons should check whether patients are on bisphosphonates and consider those taking the drug to be at some risk for ONJ.
Merck counters that "in all of our controlled clinical trials, including the 10-year data with Fosamax, which have included more than 17,000 patients, we have not had reports of osteonecrosis of the jaw occurring in patients taking Fosamax."
The Whitehouse Station, N.J., drug maker also points out that it inserted two paragraphs in the "precautions" section of the Fosamax prescribing information for doctors in July 2005 after the Food and Drug Administration requested it amend Fosamax’s label.
Salvatore Ruggiero, chief of oral surgery at the Long Island Jewish Medical Center in New York, says of the 155 ONJ cases he has come across, 22 involve patients who were taking Fosamax and other oral bisphosphonates. Some of these patients took Fosamax for seven or eight years, he says. "With the oral drugs like Fosamax, exposure time is the key," Dr. Ruggiero says.
Plaintiffs’ attorneys have seized on such findings to allege that Merck engaged in the same deceptive behavior with Fosamax as it did with Vioxx. Like Vioxx, Merck has marketed Fosamax aggressively and today has more than $3 billion in annual sales from the drug. Doctors wrote 22.4 million prescriptions for Fosamax in the U.S. last year, according to IMS Health.
Henry Bone, director of the Michigan Bone & Mineral Clinic in Detroit, says the link between oral bisphosphonates like Fosamax and jaw decay is overblown. Fosamax "and other bisphosphonates are very important drugs for the treatment of osteoporosis," he says. "Rare reports of the ‘osteonecrosis of the jaw’ syndrome do not outweigh the benefits of these valuable medications." Dr. Bone has received research funding from Merck and once worked as a paid consultant for the company.
David Tundell, a 61-year-old former aircraft maintenance officer in the Air Force and a plaintiff in the Florida suit, says the Fosamax he took for a year helped alleviate the osteoporosis in his hips. But he believes it also landed him in the emergency room earlier this year when his jaw swelled to the point where he could no longer eat. During a three-day hospitalization, all his teeth were taken out and part of his jaw was shaved off to remove dead bone. He says his doctor recommended he stop taking Fosamax.
The prevalence of ONJ is hard to determine. The National Osteonecrosis Foundation says there are 20,000 Americans a year who are diagnosed with osteonecrosis, but it doesn’t break out the number of jaw cases. The foundation says anecdotal evidence suggests there has been a surge in jaw cases of late.
Yesterday, a jury in Atlantic City, N.J. awarded $9 million in punitive damages to a 77-year-old man who had a heart attack while taking Vioxx, bringing total damages awarded to him and his wife to $13.5 million. It was the second Vioxx case Merck has lost.
"Certainly, they’re hoping for spillover" from the Vioxx cases, says John Brenner, a partner with McCarter & English in Newark, N.J., which isn’t involved in cases with either drug. "They’ll try to paint it as a matter of pattern and practice on Merck’s part. But you’re going to be hard-pressed in a trial to introduce Vioxx in a Fosamax case."
Mr. O’Brien, the lawyer with Levin Papantonio, argues that Fosamax could become a bigger problem for Merck than Vioxx because ONJ is a so-called "signature" disease that is rare among patients who haven’t taken bisphosphonates. He notes that dentists now refer to the condition as "fossy jaw" in a play on the middle syllable of the word "bisphosphonate." Levin Papantonio, which is also involved in the national litigation over Vioxx, is representing 200 patients in its Fosamax lawsuit and expects that number to rise.
Other pharmaceutical companies make oral bisphosphonates for osteoporosis. French drug maker Sanofi-Aventis SA makes Actonel and Swiss drug maker Roche AG makes Boniva. Mr. O’Brien says his firm decided to target Fosamax because data from the FDA’s bisphosphonate safety review showed a much higher rate of ONJ with Merck’s drug than with the others. "We’ll take on any big pharmaceutical company," he says. "We’re not doing this just because it’s Merck."
In the complaint it filed Monday, Levin Papantonio alleges that the FDA’s drug-safety office concluded that Merck and other bisphosphonate makers should change their labels to reflect the risk of ONJ as early as August 2004, 11 months before Merck changed the Fosamax label.
Merck counters that it submitted a draft revised label for Fosamax to the agency in March 2005. An FDA spokeswoman says it isn’t "at liberty to discuss details of internal labeling negotiations between the agency and a company." She adds that the current Fosamax label "adequately conveys what is known about ONJ-bisphosphonates."
Source: JOHN CARREYROU, for the Wall Street Journal