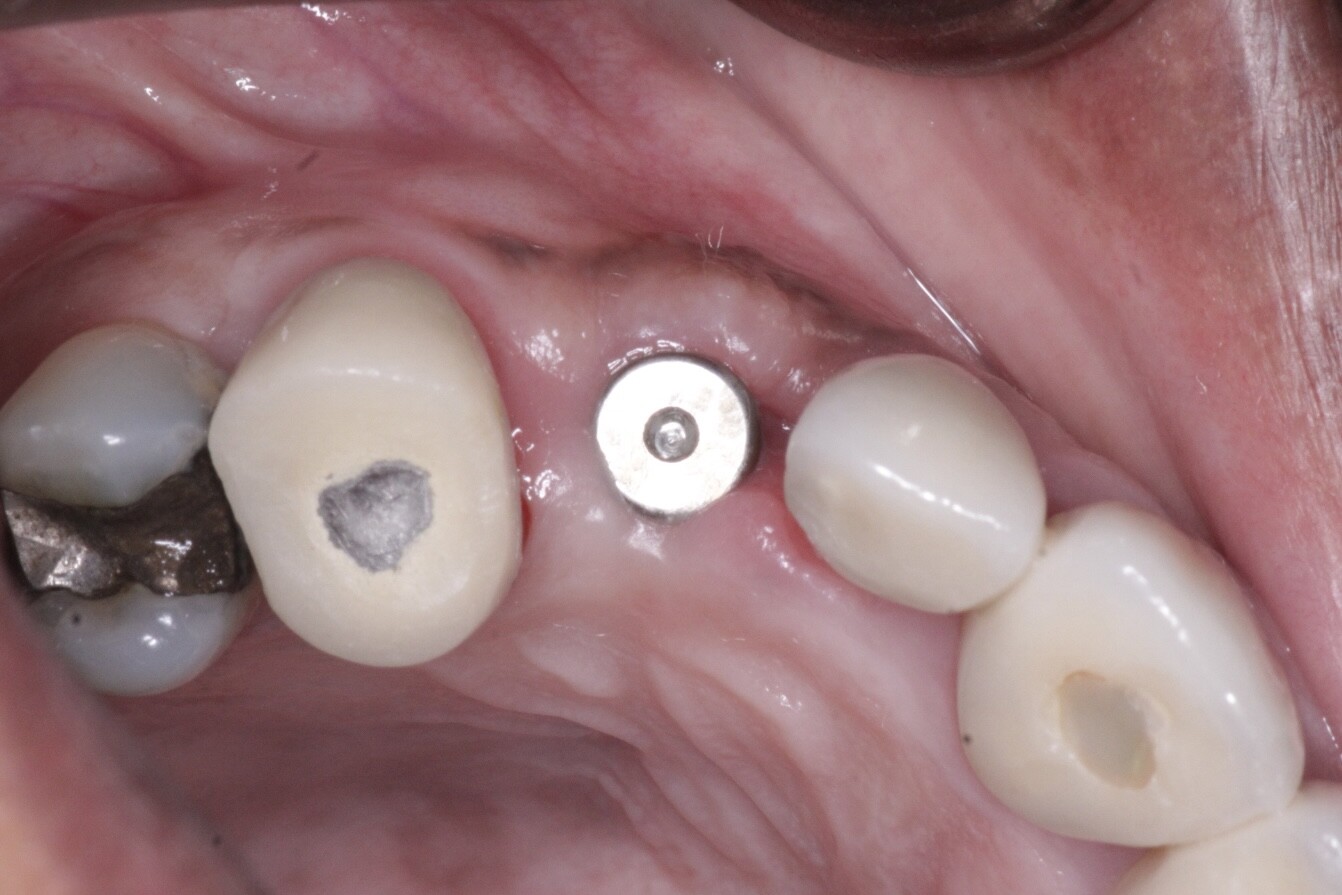
New Barrier Membrane Introduced
![]Episample](http://osseonews.blogs.com/.shared/image.html?/photos/uncategorized/episample.jpg)An improved Epi-Guide(tm) Barrier membrane is being introduced by
Curasan, recognized innovators in osseoregeneration products, to meet
clinician requests for a more malleable, less rigid
product. This easier-to-handle membrane remains the only bioresorbable
synthetic matrix on the market with a unique three-layer technology to
provide predictable healing.
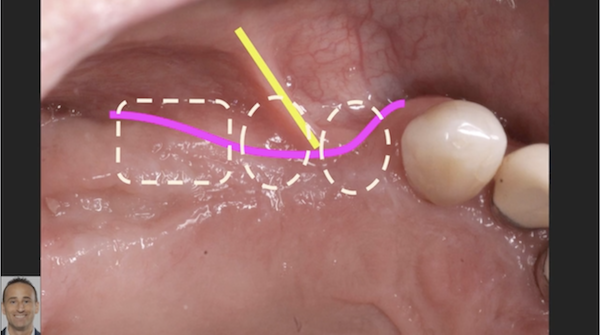
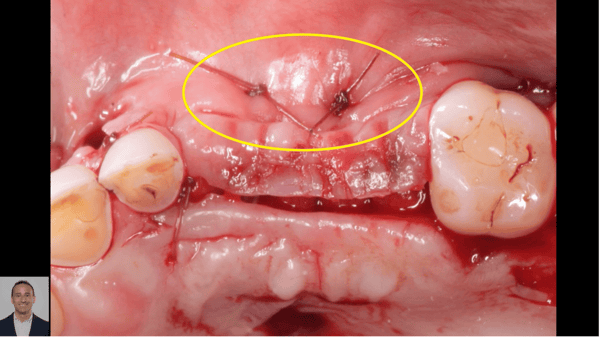
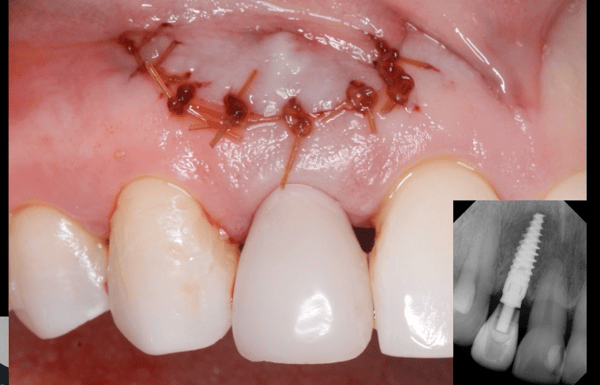
For clinicians, the extra malleability offers excellent handling characteristics. Like the original product, the improved Epi-Guide comes in a sterilized 18 x 30 mm single membrane sheet.
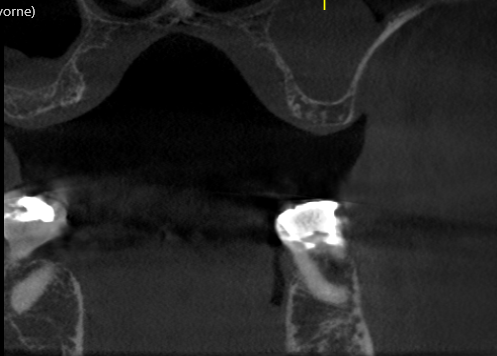
Epi-Guide – developed for adjunctive therapy in periodontal restorative surgery, as well as for Guided Tissue Regeneration (GTR) and Guided BoneRegeneration (GBR) applications – is used primarily by implantologists, oraland maxillofacial surgeons, and periodontists. Unlike competitive products
that use collagen from bovine sources, Epi-Guide is synthetic, allaying any patient concerns about the safety of animal derivatives.
Epi-Guide is made by Kensey Nash and marketed in the United States by Curasan Inc., the North Carolina-based subsidiary of Curasan AG.Instructions for use and published data about Epi-Guide and Cerasorb,Curasan’s flagship product, are available on Curasan’s expanded website at:
www.curasan.com., or by calling 888-237-2767.