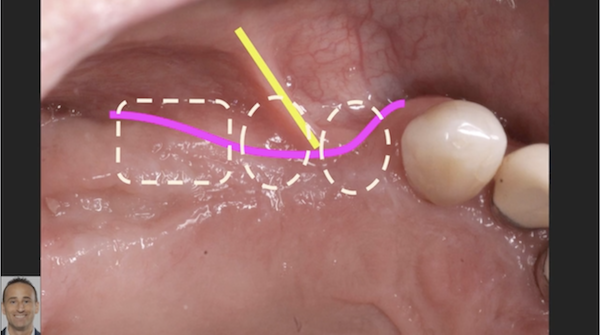
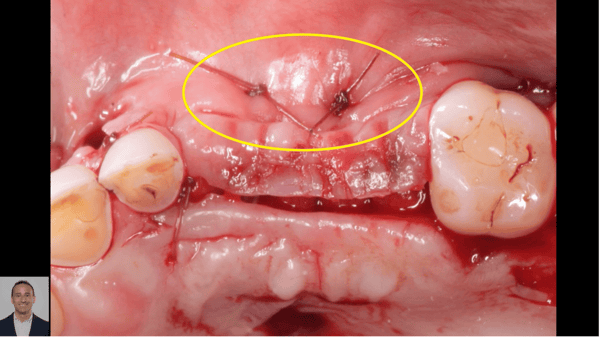
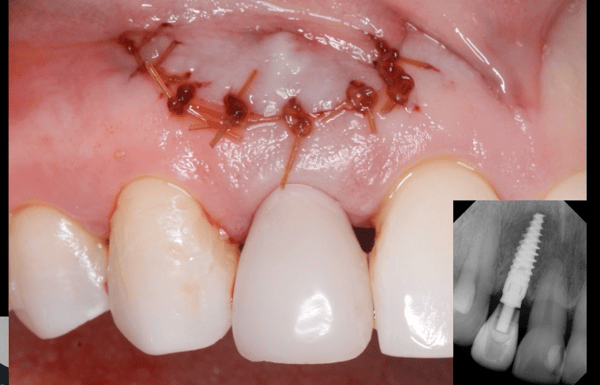
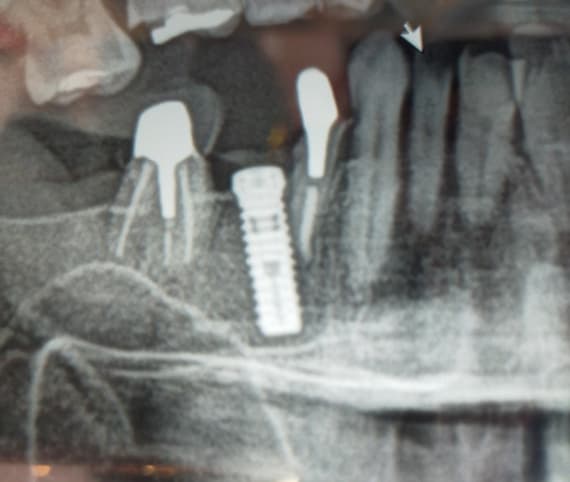
Recombinant Growth Factor for Periodontal Applications
![]Gems](http://osseonews.blogs.com/.shared/image.html?/photos/uncategorized/gems.gif)GEM 21S® (Growth-factor Enhanced Matrix) has been approved by the U.S. Food and Drug Administration (FDA). GEM
21S® is a fully synthetic regeneration system for the treatment of
periodontal bone defects and associated gingival recession.
GEM 21S® is composed of the tissue growth factor, recombinant human
Platelet-Derived Growth Factor (rhPDGF-BB), and a synthetic bone
matrix, Beta-tricalcium phosphate (ß-TCP). It is the first
totally synthetic product combining a purified recombinant growth
factor with a synthetic bone matrix to be approved by the FDA for human
application. In
recognition of the unique treatment modality provided by GEM 21S®, the
FDA has established it as a first-in-class product in its treatment
category.
Read the entire press release by clicking here. Please feel free to leave your comments below.