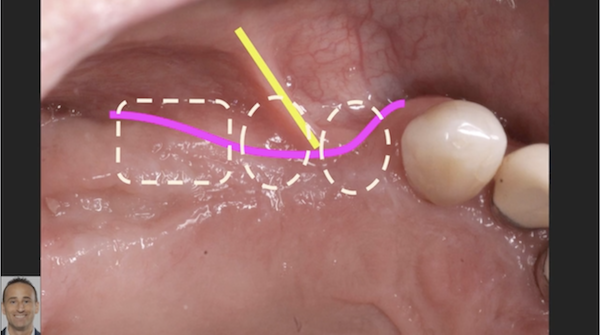
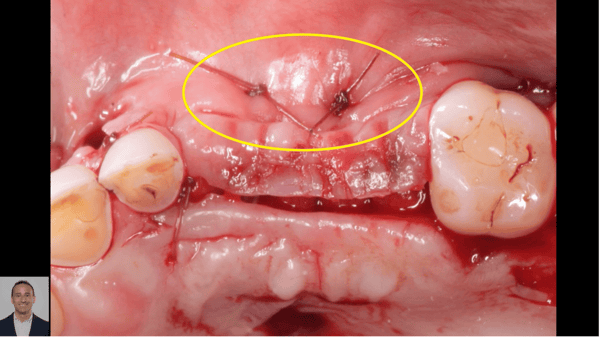
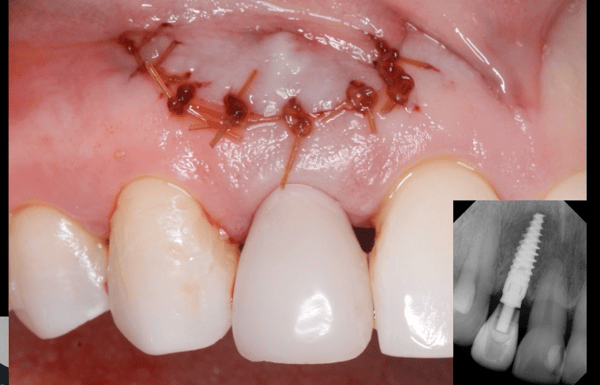
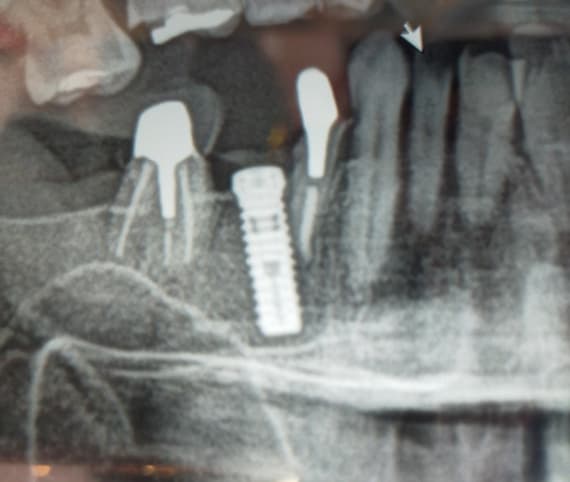
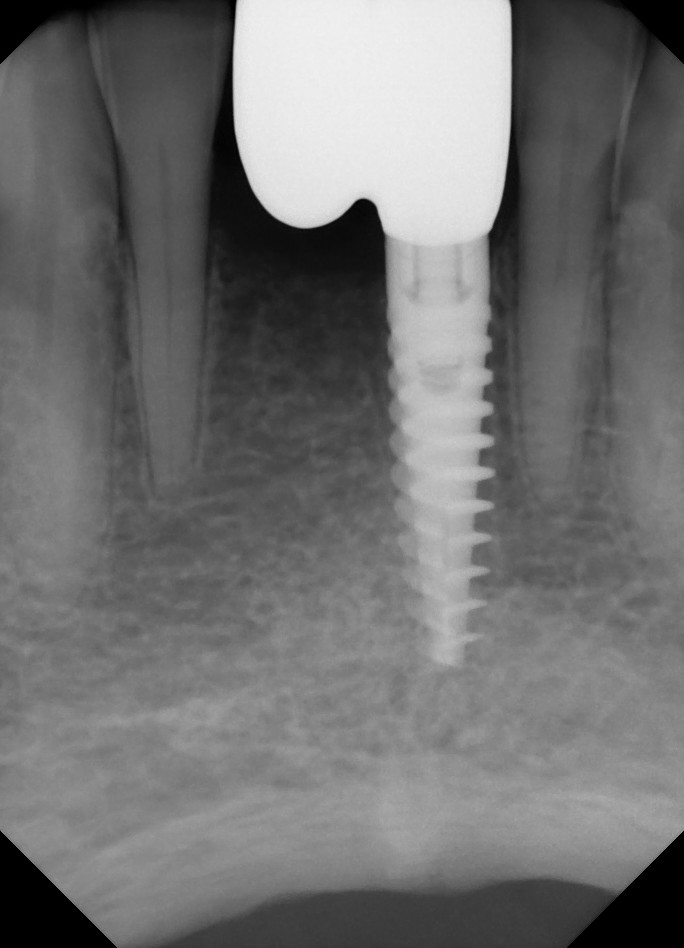
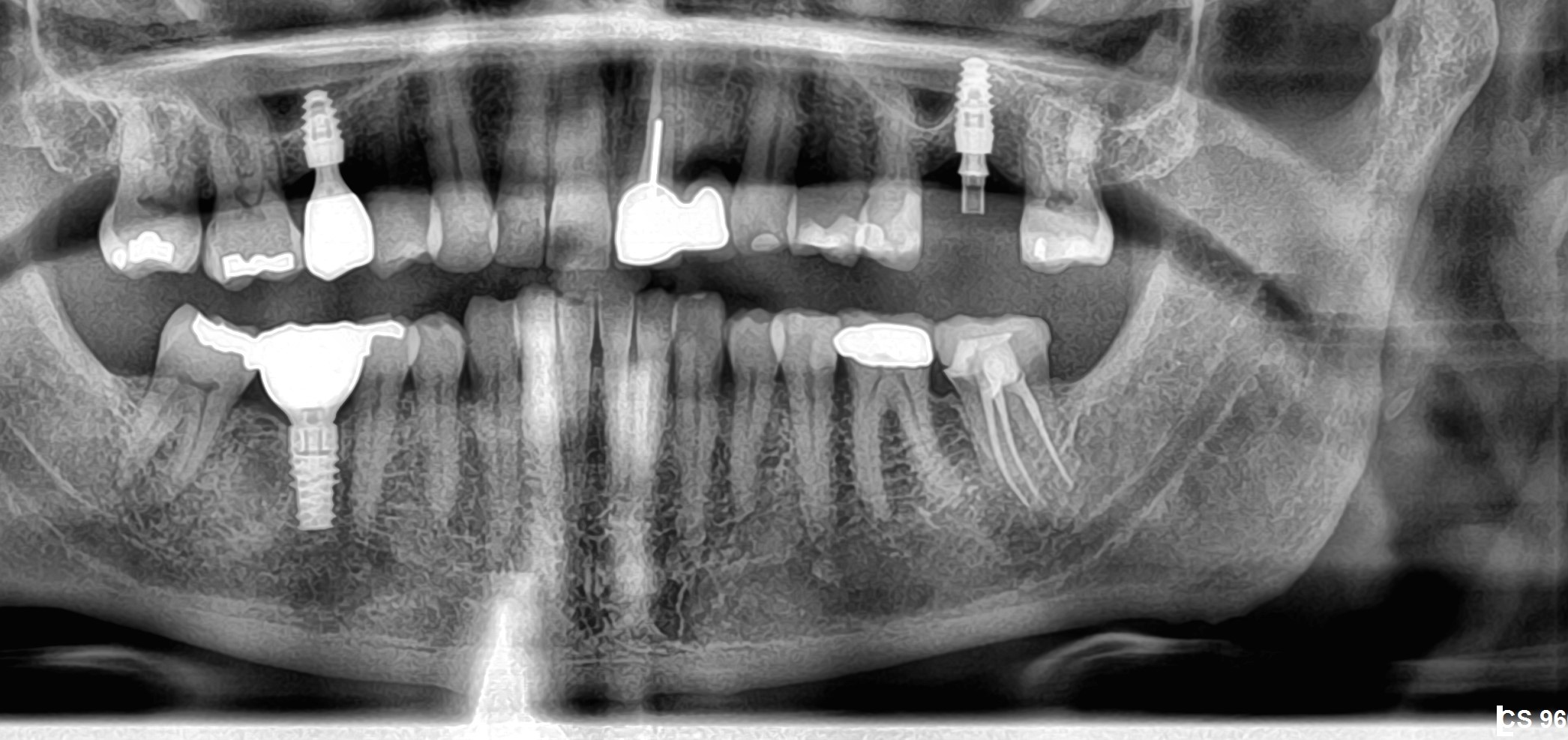
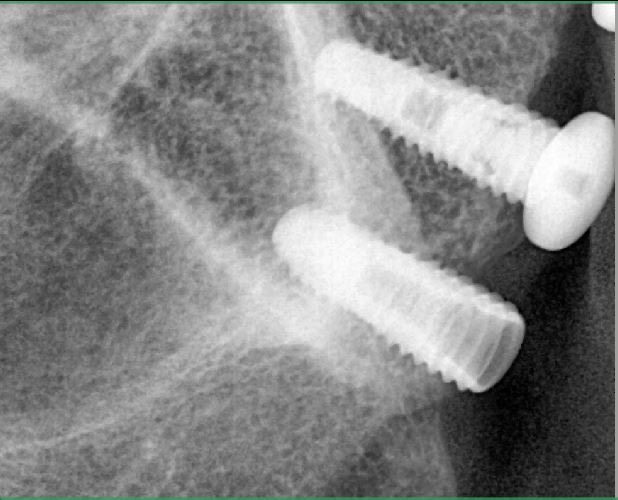
Correlation between grafting and peri-implantitis?
Allowing for the naysayers, there seems to be an exponential rise in periimplantitis over the past few years. There are so many vested interests at play, that I really do not expect to get much in the way of coherent responses, but would be interested in any observations. So far as I am aware the likely periimplantitis predisposing factors are: Implant design, Operator technique, and Case selection (e.g. perhaps treating more smokers/ poorly-treated perio). The other factor that seems to have risen exponentially is the tendency (with the best of intentions) for the great and the good to graft every case they can. Is there a meaningful correlation between grafting and periimplantitis?
36 Comments on Correlation between grafting and peri-implantitis?
New comments are currently closed for this post.
peter Fairbairn
11/6/2014
This is a great question and there is a bit of research on the matter to back the notion up ( Just saw a Dog study being presented at the EAO in Rome ) bur the main issue is what graft is used . If a fully bio-absorped material is used no issue but materials with an HA ( animal or synthetic ) have shown in studies to have an issue.
Personally I graft 60% of cases and the grafted cases tend to have better peri-implant bone over the long term often gaining 1 mm in height in the first year ....but I always use materials that are fully bio-absorped... leaving only host regenerated bone which with functional re-modeling appears to have minimal issues .
Anyway should be a good debate..
Peter
greg steiner
11/8/2014
Your observations and Peters comments are in line with what others are experiencing. If you are talking about grafting around immediate implants it has been shown that allografts and xenografts fill the void but do not integrate to the implant in the area of the graft. Also because allografts and xenografts are mineralized and never resorb you have no idea if your graft has mineralized or it is just graft particles in fibrous tissue. Studies have shown allografts and xenografts around immediate implants commonly are just graft particles in fibrous tissue which will likely become infected in time producing what you are seeing as periimplantitis. On the other had if you are talking about failing delayed implants in sites grafted with allografts or xenografts the failure is not infection but the breakdown of sclerotic bone produced by these materials. It is estimated that 25% of implant failures are due to sclerotic bone breakdown in sites previously grafted with allografts or xenografts. Greg Steiner Steiner Laboratories
DrG
11/11/2014
Great discussion: Greg, what are your thoughts regarding immediate implants? Should we go the direction of membrane and no graft? PRP?
Alejandro Berg
11/11/2014
I would have to disagree. We have been using allografts for many years as I know Peter does and there a studies that show that bifasic ceramics tend to get fully integrated into live bone (resorbable part goes and HA gets surrounded by living bone. To go no further today I placed a 4.2*16 and a 3.75*13 into areas that were subject of alveolar preservation some months ago. This would have not been possible otherwise, Our ostell measures were 90 and 80 respectively. This is normal for us and after 20 years of implants ad grafts, we loose very, very few. I have no affiliation to any company or any vested interest I just speak from experience and studying and by studying I mean that we regularly take core samples with our 3mm trephine prior to implanting and do histological sampling.
Having said that we just saw a few months ago the beautiful presentation from Dr Pindholt that shows that your "graft" ( and by that I mean your remaining Ha) barely even touches your implant in a very small percentage that is not at all statistically relevant and so your implant gets osseointegrated with live bone.
So in my opinion grafting gets you better bone in terms of height and width. Mature grafts do not predispose for peri implantitis.
Cheers
Alejandro
Peter Hunt
11/11/2014
Well, there are two modern developments that have swept through implantology over the last few years that may impact on this. First the move to rough surface implants, second the move to bring the rough surface closer to the platform of the implant.
Many have problems placing their implants deep enough to make sure the platform (and rough surface) is below the bone line particularly on the labial margin. It's also normal to see a cover screw being placed, but the primary closure of the flaps is less than adequate. This means that it's easy for the rough surface to become contaminated at the outset and this can lead to peri-implantitis.
We rarely do primary closure. We place a 4.0mm gingivaformer, then place a bone graft with collagen around this to fill any channel deficiencies, to protect the crestal bone and to act as a dermal filler. The flaps are then placed around the gingivaformer and sutured with Teflon sutures. We leave these in for three weeks.
The whole idea is to provide a "Safety Seal" around the implant for the first critical few days and weeks of healing. It seems to work, we have a very high success rate with normal implant placements and using modifications of this procedure we are achieving very high success rates with immediate molar replacements. With these there is much more implant exposure, needing more bone graft and there is little or no ability to close over the region with gingiva. Instead we cover the region with Mucograft.
Robert J. Miller
11/11/2014
Good points made by all. But there is one defining factor when discussing any predisposition for future peri-implantitis when using a biomaterial, especially those that resorb more poorly. Is the graft placed at the time of implant placement or is it placed in a grafted ridge that has had time to incorporate the particulate? In my clinical experience, especially in the last few years when it seems that my practice has become the repository for many of the failed implant cases in my community, the highest percentage of peri-implantitis is in the group of immediate implant placement where the gap has been filled with a higher crystallinity HA. Salivary contamination of the crestal 1-2 mm of graft, and a resultant downgrowth of fibroblasts, virtually guarantees some type of untoward outcome, whether it is crestal bone loss or soft tissue attachment to the roughened body. Fibrous encapsulation of the coronal few millimeters of the implant body, with migrating particulate, results in a far greater propensity for breakdown, both early and late. In virtually every reentry surgery in a grafted site with simultaneous implant placement, I am curretting unincorporated graft particles, with xenografts being the worst offenders. I would prefer autogenous bone (harvested during the drilling process), followed by beta TCP hydrated in serum from PRF, or an allograft also hydrated with serum. The graft I will not include in my protocol is any high crystallinity HA, xenograft included.
RJM
greg steiner
11/12/2014
Dr. G
We need the entire surface of the implant integrated with bone if we can expect an immediate implant to be as successful as a delayed implant. The problem is that none of the traditional graft materials produce integration to the implant in the area of the graft. The graft may mineralize but it will not integrate to the implant surface. As a result the immediate implant has less support where it needs it the most. No graft-membrane fails to produce integration in the area also. This was shown by Becker and Urist where they placed titanium screws into extraction sockets in humans and then grafted the defects and then cut them out with bone and found no integration with autografts, allograft and xenografts. I do a lot of immediate implants but then our graft material is the only graft material proven to produce integration at the graft site. Greg Steiner Steiner Biotechnology
DrG
11/13/2014
Fascinating Greg,
Why is your graft material the only one that works? What exactly is in it?
greg steiner
11/13/2014
Dr. G
What determines if you get integration at the graft site is determined by what cell arrives first at the implant surface. If a fibroblast arrives first you get collagen deposition on the implant and no integration. If an osteoblast arrives first you get integration. If there is no graft you have the same process that occurs in an extraction socket- fibrin clot, then granulation tissue then collagen formation then bone. In this process you will not get integration because the fibroblasts arrived first. If you add a granular bone graft you have the same process and you do not get integration. Our graft material allows osteoblasts to crawl through the graft material but not fibroblasts and when the osteoblasts arrive at the implant surface integration occurs. It also helps that our grafts are osteogenic and the osteoblasts are stimulated to proliferate and migrate through the graft material. Greg Steiner Steiner Laboratories
Dr.Dr.Hossam Barghash
11/13/2014
Dr.Steiner. you did not supply any scientific explanation how a graft material will allow osteoblast and not fibroblast reaching the implant surface,?
peter Fairbairn
11/14/2014
Both differentiate from the mesenchymal cells and here graft stability ( in correct site ) is the key for the move to Osteoblasts..
There is lot of new research in Bone physiology circles showing alloplastic host up-regulation ( ZHao , Watanabe et al , BONE ).
Peter
DrG
11/14/2014
Dr Steiner,
I checked out your website which was really interesting. I'm still curious what exactally is in your grafting products. I don't see any scientific citations on your website that are specific to your products. I have to imaginge you have FDA approval is there a FDA citation you could provide?
Your material for sinus grafts is very intriguing especially.
greg steiner
11/14/2014
Dr.Hossam Barghash
Not explaining the science behind graft site integration is intentional. It took us years to figure it out and we don't share this information. Sorry Greg Steiner Steiner Biotechnology
greg steiner
11/15/2014
Dr. G
Yes our products are FDA approved. On the publication tab on our web site you will find an article that was published in the compendium- "The healing socket and socket regeneration.' That article was essentially the material we submitted to the FDA for approval of Socket Graft. Also if you are interested we send to our customers regular emails on bone, bone grafts and bone grafting and if you are interested call the office and ask to be placed on the email list. Greg Steiner Steiner Biotechnology
Robert J. Miller
11/16/2014
Alot of claims here, but no perspective. Three basic pathways necessary for bone growth following graft placement; space maintenance, conversion of stem cells to osteoblasts, and vasculogenesis. Over time, almost all grafts perform these three tasks. However, some are better in the earliest phases. For example, L-PRF is demonstrably the best agent for vasculogenesis and conversion of stem cells to osteoblasts. But is is a relatively poor space maintainer if you are missing key walls. Particulate grafts are better space maintainers but, depending on porosity and resorption time, may actually slow down new bone growth. Calcium sulfates are great for stem cell conversion because of the release of free ionic calcium, but have no porosity and delay early angiogenesis. So your choice of grafts should be SITE SPECIFIC, not based on what you happen to have in your inventory. The utilization of graft materials should be based on the concept of "least produces the most", meaning the material that best addresses the three parameters for bone growth in the least amount of time, and gives you the best tissue quality (percentage of vital bone). I always start with L-PRF, and then may add other graft materials to solve a particular problem in that site. But my additional graft materials are highly resorbable and potentiate the other pathways as quickly as possible. For example, I use alot of betaTCP's as my filler. They release free ionic calcium, maintain space, and are completely gone at final bone maturation for the highest percentage of vital bone. But if I need to maintain space for a longer time (one-walled defects), I will use a collagen containing allograft. Start getting used to a more biologic perspective to control your final clinical outcomes.
RJM
DrT
11/16/2014
RJM...excellent post...could you mention one or a few specific collagen containing allografts that you use. Also, do you ever mix allograft or collagen containing allograft with beta TCP? Thank you.
DrT
Robert J. Miller
11/16/2014
Any demineralized bone matrix material from a tissue bank contains collagen (i.e. Puros). Each type of demineralized allograft may vary in it's collagen ratio, but you only need a small amount to stimulate osteoblast metabolism. There are also some alloplastic products that contain engineered collagen so they can be very effective. So the following is my strategy: 5-walled defect is L-PRF alone (ALWAYS); 4-walled defect is L-PRF alone if it is a single intra-tooth space and L-PRF mixed with alloplast (bTCP) if no teeth adjacent; all other defects will be L-PRF with alloplast or allograft (or mixed). The fewer the walls, the longer I need the graft to hang around as a space maintainer, hence the move towards allograft.
RJM
Dr.Dr.Hossam Barghash
11/18/2014
I do agree with Dr.miller in his strategy, but I do not agree with Dr.G. because we are talking about biology and not scientific secret of graft. biology of graft healing need the fibroblast and endothelial cells for the angiogenesis.. . ..
greg steiner
11/20/2014
Dr. Miller
You state demineralized allografts stimulate osteoblast metabolism. This would imply allografts are osteogenic but they are not so if the allograft is not stimulating bone growth what is the use of stimulating osteoblast metabolism? Greg Steiner Steiner Biotechnology
Robert J. Miller
11/21/2014
Greg; Not sure you are using the correct terminology. If we discuss successful bone grafting, include osteoconduction (guiding reparative growth of bone), osteoinduction (encouraging undifferentiated cells to become osteoblasts), and osteogenesis (living bone cells in graft material contributing to bone remodeling), osteogenesis only occurs with autografts. Therefore, allografts are not osteogenic.
RJM
Peter Fairbairn
11/22/2014
Yes , being in the UK I find the abuse of the English language interesting....... GBR,
Guided Bone Regeneration can only be achieved by using a fully bio-absorbed particulate material . As if there is any remnant material it is not technically BONE , ask any 1 st year histology student and as for regeneration if the original state is not achieved then it cannot be regeneration maybe replacement ???
So maybe we need a more correct terminology when using materials that are not turned over fully ...... maybe Guided Bone-like Replacement ......conveniently it can thus also be called GBR....
Regards
Peter
Greg Steiner
11/24/2014
Robert
You state osteoinduction is (encouraging undifferentiated cells to become osteoblasts)
Sorry but your definition is wrong. Osteoinduction is the ability of a material to grow bone outside of bone - nothing more and nothing less. By definition osteoinduction never occurs in bone.
You state osteogenesis (living bone cells in graft material contributing to bone remodeling), This statement has nothing to do with osteogenesis
Osteogenesis is simply the ability of a material to make bone grow faster than normal. Osteogenesis requires something in the material to modify the osteoblasts physiology to make it produce bone faster. I should know this term as our bone grafts are the worlds only osteogenic bone grafts. Autografts do not stimulate bone to grow faster and are not osteogenic. I agree allografts are not osteogenic as they only produce mineralized scar tissue. I hope this helps. Greg Steiner Steiner Biotechnology
Robert J. Miller
11/24/2014
osteoinduction, (os´tÄ“Åinduk´shn),
n the process by which undefined cells of loose makeup undergo mitosis and form osteoprogenitor cells.
Mosby's Dental Dictionary, 2nd edition. © 2008 Elsevier, Inc. All rights reserved.
Sorry Greg, but your definition is a bit off the mark. I will give you this; the proof of osteoinductivity in the literature IS the ability to grow bone ectopically. But the means by which this occurs is codified by the preceding descriptor. With regard to osteogenesis, only live bone producing cells can in turn create additional bone. We know that grafts containing BMP's are osteoinductive, but in the orthopedic literature a calcium phosphate material is also considered to be osteoinductive. I posted these references in another Osseonews blog a couple of years ago. Don't know what it is about your new graft material that seeks to upend conventional biology. Perhaps you could share it with us.
RJM
peter Fairbairn
11/25/2014
As I said in my first post this will be a GOOD debate , again it is afflicted by terminology as the Dental world ( or industry ) sees things differently from the Orthpedic , Spinal and Bone physiology worlds ( everyone else ) when it comes to bone .
BTcP is osteo-inductive ( See Zhao, Watanabe et al BONE 2009 , Elsivier ) or my animal study ( Implant Dentistry , Feb 2014 , ICOI ) , we are currently completing a very comprehensive animal study ( 1 of 3 current studies ) which merely concurs but in a compelling manner . Up to 3,500 genes for increased Osteoblast presence were noted in the Zhao study ( a must read if interested in Bone ) which is interesting .
We have also noticed this in clinical cases and multi-centre studies from patient core sample analysis from some of over 2,500 graft cases .
That is merely the science , as for terminology , interpret it which ever way we feel helps us and our patients .
Regards
Peter
greg steiner
11/26/2014
Robert
One problem with the definitions is how our knowledge has changed over time. The definition of osteoinduction "the process by which undefined cells of loose makeup undergo mitosis and form osteoprogenitor cells" was created before we knew about stem cells. By this definition all bone growth is osteoinduction. One thing we agree upon is that the test for osteoinduction is the ability of a material to form bone outside of bone. However ectopic bone formation does not involve any cells of the osteogenic lineage. Ectopic bone formation does not involve mesenchymal stem cells, osteoblast precursers or osteoblasts. In muscle the bone formed is produced by reprogramed endothelial cells. In arteries the bone formed is produced by reprogramed smooth muscle cells. No bone formed outside of bone has anything to do with osteoprogenitor cells so the test makes the definition in Mosby's dental dictionary impossible. The FDA has clearly informed me that a material is osteoinductive if it produces bone outside of bone.
Osteogenesis means bone formation and of course only cells can form bone but the term is not used alone. If a material stimulates bone formation above normal than you can claim that the material stimulates osteogenesis. All studies dealing with osteogenesis are looking at the ability of a material to form bone at a faster rate with higher mineralization than normal. The FDA does not care about the mechanism. You state that only autografts are osteogenic (stimulates osteogenesis) because they contain living cells. The problem with this is that there are never any living cells in a bone autograft. In an autograft all cells necrose due to lack of blood supply. No bone cells survive the transplantation so this definition is impossible. Our materials do not change biology but work with the normal bone growing process producing bone that is formed faster and contains more mineralized tissue than normal but the bone is normal is every other way. Our graft materials are the only graft materials in the market that enter the cell and change the physiology of the cell to stimulate osteogenesis. Our bone grafts are classified as a drug and as a drug it must be safe and effective. If it was not effective in stimulating osteogenesis it would not have been approved by the FDA for that purpose. Osteoinduction, osteogenesis and osteoconduction are the few terms we have to discuss how bone grafts work. I feel it is worth our time to clarify the meaning of these term in order to communicate effectively. Greg Steiner Steiner Biotechnology
David Corbin
7/15/2015
Greg Steiner wrote: "Our bone grafts are classified as a drug and as a drug it must be safe and effective."
Please tell us the name of your bone graft, so we can look it up in the FDA drug database at:
http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Addlsearch_drug_name
Thank you.
Robert J. Miller
11/26/2014
Greg; I am curious as to why you make the statement that no cells survive in an autograft. If that is true, then all stem cell harvesting procedures in combination with a graft are nonsense. It would also mean that other autologous biologics, such as L-PRF, are also ineffective. But we know from the literature that these cells do, in fact, survive and contribute growth factors to a graft milieu for hours or even several days. How do we then reconcile our differences in opinion?
RJM
greg steiner
11/26/2014
Robert
I specifically stated that all cells become necrotic in a bone autograph.
Greg Steiner Steiner Biotechnology
Richard Hughes, DDS , FAA
11/27/2014
Robert ,
You make a good point. Autografts are the "Gold Standard".
Greg,
Do you have published data to support the statement that your graft material enters cells and changes the physiology of the cells and stimulates osteogenesis! That's a bold statement.
Enters what cells?
greg steiner
11/29/2014
Richard
The information on the cellular activity of our bone grafts are a result of many different experiments that we presented to the FDA. The results are condensed and listed below and on our Socket Graft Putty web site. However citing the molecular activity is interesting but worthless in regard stimulating osteogenesis. Growth Factors for example produce interesting metabolic reactions but do not stimulate osteogenesis primarily because they are only active for a short time.. The reason why our material is the only graft material that stimulates osteogenesis is because it enters the cell and stimulates the osteoblast but more importantly it is stored in the osteoblast and stimulates the osteoblasts for months. Stimulating osteogenesis is a histological finding and we have published our histology in the Compendium. The complete article can be found on the publications tab on our web site named The Healing Socket and Socket Regeneration. Our grafts produce mineralized tissue faster than normal and continue to produce mineralization until the bone is 90% mineralized which has never been accomplished by any other graft. But it is not mineralization that we are interested in because it does not produce integration. It is the stimulation of osteoblasts in our graft material that results in 100% integration rates and 100% implant success over time. Because we stimulate the normal process of bone formation the bone produced by our grafts functions as normal bone which is critical to long term implant success. If you want confirmation that our compound enters that cell and is classified as a drug contact me through our company email and I will send it to you along with some histology you would find interesting. Greg Steiner Steiner Biotechnology
SL Factor enters the through the osteoblasts’ membrane and activates over 300 genes that stimulates the process of bone formation.
Stimulates mesenchymal stem cells to differentiate into osteoblasts.
Creates a two-fold increase in BMP2 and Runx2 production and reduces osteoclast formation by inhibiting the production of RANK ligand.
Enhances osteocalcin accumulation in the extracellular matrix resulting in excellent mineralization.
Stored in the osteoblast and continues to stimulate bone growth after the synthetic matrix has been resorbed.
Inhibits adipogenesis, osteoclastogenesis, and ODF/RANK ligand expression in bone marrow cell cultures.
greg steiner
11/29/2014
poster
I would like to apologize that your post has gotten hijacked into a different direction but your observation is very insightful. Greg Steiner Steiner Biotechnology.
Robert J. Miller
11/30/2014
Greg; the list of actions of your biomaterial sounds an awful lot like autologous biologics. But there is no graft material that behaves like platelet rich fibrin. L-PRF is fibrin based and is the naturally optimized carrier for delivery of tissue growth factors. This material releases high quantities of three main growth factors at >7 days, partly by the blood components (platelets) and by a chemotactic effect on cells also producing growth factors such as: Transforming Growth Factor beta-1 (TGFb-1, Platelet Derived Growth Factor AB (PDGF-AB), and Vascular Endothelial Growth Factor (VEGF). These cytokines stimulate cell replication, promote angiogenesis, epithelialization, and formation of extracellular matrix (granulation tissue). They regulate bone cell metabolism and increase alkaline phosphatase activity by over 800% in the early phase of bone growth. PRF also controls differentiation of mesenchymal stem cells in target tissues. Also released is interleukin-4. This cytokine supports the proliferation and differentiation of activated B-lymphocytes. During the inflammatory process, its principal function is to moderate inflammation.
One thing that no bone grafts contain is a leucocyte component. White blood cells are present in L-PRF and play a key anti-infectious role, both in direct phagocytosis and immune regulation. Monocytes can perform phagocytosis using intermediary (opsonising) proteins such as antibodies or complement that coat the pathogen, as well as by binding to the microbe directly via pattern-recognition receptors that recognize pathogens. Monocytes are also capable of killing infected host cells via antibodies, termed antibody-mediated cellular cytotoxicity.
I will use L-PRF (IntraSpin System) in virtually all of my surgeries, usually alone but occasionally mixed with a graft material if I have to maintain a space. We have cored these sites, where PRF is used alone, to do the histomorphometry and am amazed at the mineralization and numbers of bone cells present. There are papers that cover the gene sequences that are upregulated including micro DNA analysis. While I will agree that there are biomaterials that can significantly outperform other graft materials, there are none that surpass autologous biologics.
RJM
Richard Hughes, DDS , FAA
12/1/2014
Greg,
I visited your company website.
What are the constituents of the bone cement and wetting agent?
greg steiner
12/1/2014
Robert
Your response is timely as I have been working on an email to our customers titled Understanding Growth Factors. I reviewed every publication listed on PUBMED that involved platelet rich fibrin. According to PubMed there are 16 controlled human clinical studies on PRF and one controlled animal study.
There were 4 human sinus augmentation studies all of which compared PRF mixed with either bovine bone or allograft and compared this to using the bone graft alone. All studies showed no benefit by adding PRF. One sinus study compared grafting the sinus and compared covering the graft with a PRF membrane to covering the graft with a collagen membrane. The study showed no benefit gained by using the PRF membrane over the collagen membrane.
There were 5 human periodontal studies comparing PRF to open flap debridement. All studies showed PRF superior to open flap debridement. One periodontal study compared PRF mixed with Allograft and found it better than allograft alone. One periodontal study compared PRF mixed with bovine bone and found PRF mixed with bovine bone better than PRF alone.
There were 4 studies on socket healing. Three studies compared PRF to no socket graft and found no difference between between the sockets grafted with PRF and no grafting and one study showed PRF performed better than no graft.
From these studies PRF clearly shows no benefit in the sinus. It shows no benefit as a membrane. In the periodontal studies PRF was compared to open flap debridement which is not a regenerative procedure. Why did none of the periodontal studies compare PRF to simple bone grafting or GTR. They probably did and did and did not like the results.
For socket grafting the majority of the studies show no benefit with grafting with PRF. But again they never compared to socket grafting with bone grafts.
Probably the most interesting study was a well done study in dogs studying extraction sockets grafted with PRP, PRF, no graft and platelet poor plasma that has the growth factors removed. Platlet poor plama out performed PRP and PRF and no graft. The authors concluded that growth factors in PRP and PRF inhibited bone formation. This makes sense because the growth factors in these preparations are not associated with bone formation. Another interesting fact was obvious from this review and that none of the studies were done for orthopedic bone growth. Apparently they know something that we don’t. In my opinion there is no scientific support for using PRP or PRF for bone regeneration. Greg Steiner Steiner Biotechnology
Robert J. Miller
12/1/2014
Greg; I find your conclusions to be either biased or misinformed. So let's respond to your post point by point. With regard to sinus grafting, you state that there is no benefit to adding PRF to a graft material. But you did not quote any of the papers where PRF was used alone. The histology of these sites was superior to grafted bone whether looking at mineralization or osteocyte count as there was no residual graft material. With regard to the use of a PRF membrane compared to collagen membrane over the sinus access, you state that there was no benefit. But there was no detriment as well. The point here is you do not have to absorb the cost of collagen membranes when you get a PRF membrane at a fraction of the cost and it prevents fibroblast infiltration just as efficiently. Next, whenever you see a regenerative site in periodontal surgery where PRF is mixed with a graft, it is often necessary to use a graft in less than a five-walled defect, as PRF is too biologically active and won't maintain a space long enough to form the desired volume of bone. There are several new papers on their way to being published that will show outstanding regeneration in sockets with PRF alone. And what version of PRF were they using? Was it the standard L-PRF protocol, or was it CGF, A-PRF, PRGF or some other iteration with different centrifuges? The efficacy of PRF is DIRECTLY related to the number of vital cells in the fibrin matrix. One of the papers in the pipeline will show dramatic differences in cellularity just based on centrifuge vibration alone. Does the study have a sample with vital monocytes. At 450g centrifugation (L-PRF protocol), monocytes are activated and release BMP's. Would you agree that BMP's are inductive growth factors? Last, we get far faster epithelialization over open wounds when using PRF as compared to collagen membranes (presence of PDGF and FGF). So not all PRF's are created equal, as not all bone grafts are created equal. While I certainly remain a devotee of the literature base, I have always used it with a critical eye and will not hesitate to be question their scientific method. At the end of the day, my clinical outcomes are paramount. Unquestionably, my results are vastly superior when PRF is employed.
RJM
greg steiner
12/1/2014
Robert
I have very much enjoyed discussing these topics with you. It is always educational to share ideas with someone who knows this subject as well as you do. Thank you. Greg Steiner Steiner Biotechology