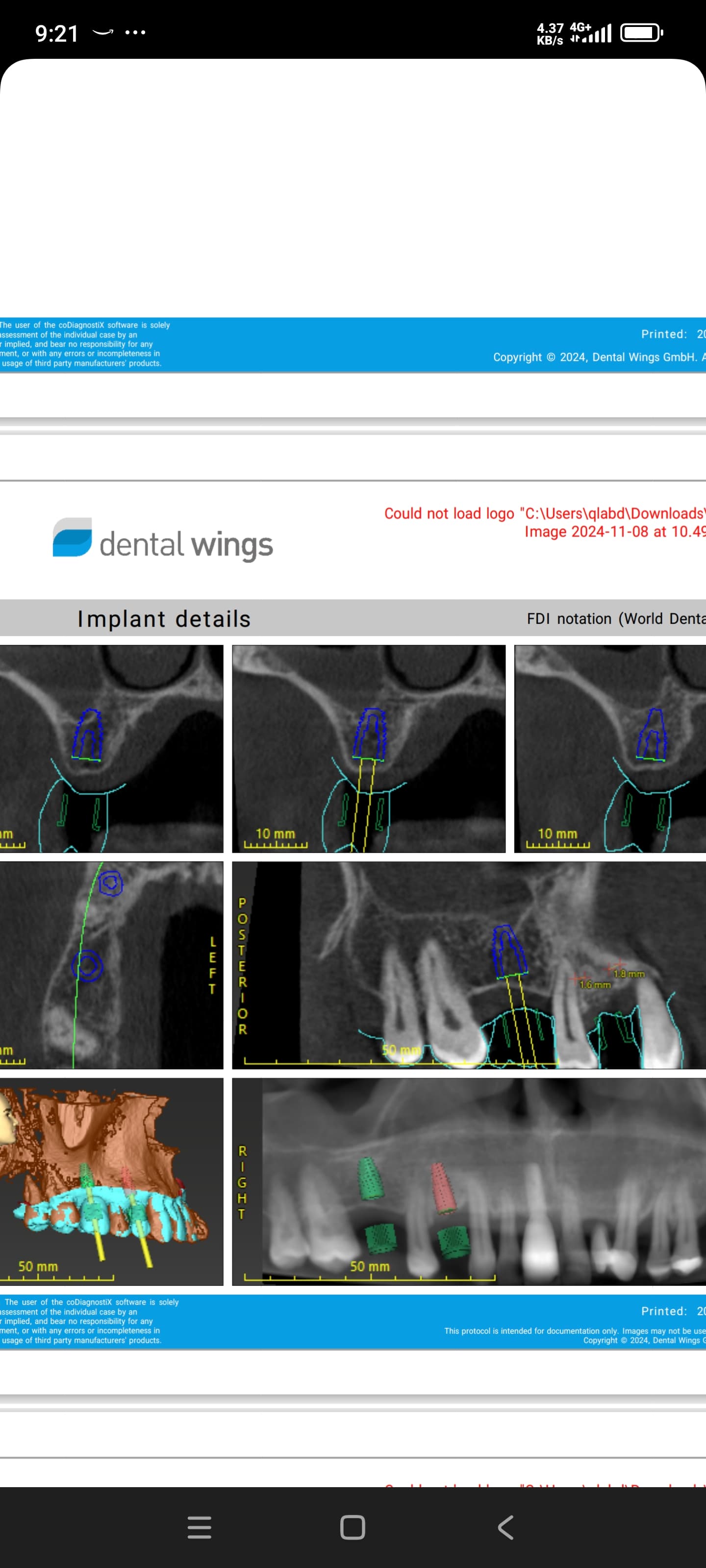
Jaw Decay Linked to Fosamax
Source: By Linda Marsa, Special to The LA Times
Sue Piervin never suspected the pills she took to strengthen her bones
could severely damage her jaw. Twelve years ago, a routine X-ray
revealed her bones were thinning, so her doctor prescribed a drug to
help stop the erosion of bone density. Then, in 1999, Piervin developed
a painful bone spur in her jaw that had decayed to such an extent that
it had to be surgically removed.
At
the time, doctors were puzzled. But when she had a recurrence last
year, they had a pretty good idea what was causing the trouble:
Fosamax, the medication she was taking to prevent bone loss.
Since
2001, more than 2,400 patients taking Fosamax and other bone-building
medications like it have reported bone death in their jaws, mostly
after a minor trauma such as getting a tooth extracted. Most were
taking especially potent, intravenously delivered versions of these
drugs, which are known as bisphosphonates.
An additional 120
people who were taking bisphosphonates in pill form to prevent bone
thinning have been stricken with such incapacitating bone, joint or
muscle pain that some were bedridden and others required walkers,
crutches or wheelchairs.
The incidence of both these
complications is minuscule in comparison with the millions of people
taking these medications. More than 36 million prescriptions for oral
bisphosphonates, such as Actonel, Fosamax and Boniva, were dispensed in
2005, according to IMS Health, a pharmaceutical information and
consulting company. Nearly 3 million cancer patients have been treated
with intravenous versions of the medications.
But because at
least 90% of drug side effects aren’t reported to the Food and Drug
Administration, the real number of people stricken with jaw necrosis
and other side effects could be higher.
"We’ve uncovered about
1,000 patients [with jaw necrosis] in the past six to nine months
alone, so the magnitude of the problem is just starting to be
recognized," says Kenneth M. Hargreaves, chair of the endodontics
department at the University of Texas Health Science Center in San
Antonio.
With concern growing over the possible side effect, the
American Assn. of Endodontists last week released a position statement
on the problem. "Until further information is available, it would
appear prudent to consider all patients taking bisphosphonates to be at
some risk," the group said.
Unreported cases of the pain
syndrome may be "considerable," says Diane K. Wysowski of the FDA’s
Office of Drug Safety, "because physicians may attribute the pain to
osteoporosis."
The issue is especially worrisome, says Dr. Susan
M. Ott, an osteoporosis expert at the University of Washington in
Seattle, because the number of women taking bisphosphonates stands to
increase now that women are more reluctant to preserve their bones by
taking estrogen after menopause.
In 2002, when a landmark study
revealed that hormone replacement therapy carried slight but measurable
heart and breast cancer risks, prescriptions for oral bisphosphonates
shot up 32%, according to IMS Health.
Bisphosphonate drugs
have been used since 1995 to strengthen bone in women who are losing
bone density and for nearly 15 years in men and women who have cancer.
The medicines act by altering the dynamics of bone, which is constantly
being turned over.
Cells called osteoclasts break bone down.
Others called osteoblasts build it up. Osteoporosis occurs when
formation of new bone does not keep pace with bone destruction.
Debate over risks
Bisphosphonates
thwart the action of the osteoclasts, thickening bones and making them
less likely to break. Physicians aren’t sure why these drugs sometimes
do seemingly the opposite and cause jaw death. But they know that
osteoclasts are also involved in prompting osteoblasts to form.
Consequently, over time, these medications may actually impede rather
than promote the creation of new bone.
Christopher Loder, a
spokesman for Fosamax maker Merck, points out that osteonecrosis of the
jaw with Fosamax is "exceedingly rare." "In all of our controlled
clinical trials with Fosamax, which involved more than 17,000 patients,
including some that were 10 years in duration, we had no reports" of
it, he says.
The risk appears to vary according to the strength
of the bisphosphonate being used. Recent studies show that about 80% to
90% of jaw decay occurs in cancer patients who take potent intravenous
bisphosphonates (Aredia, Zometa). The drugs replenish bone tissue that
is lost when cancer spreads to the bone and can reduce pain and the
risk of debilitating fractures.
The rare side effect, called
osteonecrosis of the jaw, causes severe infections, swelling and the
loosening of teeth. Patients often require long-term antibiotic therapy
or surgery to remove the dying bone tissue.
"I’ve taken off
several jaws because of this problem," says Dr. Salvatore Ruggiero, an
oral surgeon at Long Island Jewish Medical Center in New York who was
among the first to observe this phenomenon in 2001. "Because bone death
can’t be reversed, there’s nothing we can do for these patients except
ease their pain and prevent it from spreading."
Patients who
have cancer-related bone weakening and pain have few options but to
take bisphosphonates. More worrisome for experts are the millions of
women such as Piervin who take the weaker bisphosphonate pills to treat
osteoporosis, and for many more years than do cancer patients. "Even
though the chances of getting this are small, considering there are 23
million women taking this drug, we could be talking about a significant
number of people," Ruggiero says. "Risks increase the longer you’re on
the drugs, and it can take years for the complication to manifest
itself."
It’s not uncommon for rare side effects to come to
light only after a drug has been approved, says Dr. Eric Colman of the
FDA’s Division of Endocrine and Metabolic Drugs in Silver Spring, Md.
Serious adverse reactions that weren’t apparent in premarket tests
emerge in half of all prescription medications.
"People need to realize there are unknown side effects with every drug, and these medications are no exception," he says**.**
What patients can do
In
the last two years, drug makers have added warnings about bone death to
some of the medications’ labels and about the pain syndrome to all of
them.
But despite an alert sent to physicians by the FDA in
2004, "it’s been a battle getting people educated," Ruggiero says.
Dentists and oncologists know about the problem, but gynecologists and
family doctors, who write many of the prescriptions for oral
bisphosphonates, aren’t as informed.
Patients
need to be vigilant. "Women taking these drugs for osteoporosis should
tell their doctor if they develop severe pain," says Dr. Theresa Kehoe,
an endocrinologist with the FDA’s Division of Endocrine and Metabolic
Drugs.
In addition, anyone who uses oral or intravenous
bisphosphonates should alert their dentist and oral surgeon if they
need an invasive dental procedure. Better yet, says Hargreaves, get
dental work done before going on these drugs, although avoiding jaw
trauma is no guarantee of protection.
The drugs greatly reduce
risks of incapacitating fractures for older women with osteoporosis.
Women who don’t have osteoporosis but have other risk factors, such as
usage of bone-depleting steroids, previous fractures or a family
history of the condition can also benefit, says Dr. Charles H. Chestnut
III, who heads osteoporosis research at the University of Washington.
But
they should be considered far more cautiously by younger women who have
less bone thinning and are taking oral bisphosphonates simply to
prevent further deterioration. These meds become incorporated into the
bone’s matrix, where they can linger for five years or more. Their
effects are cumulative. And women are expected to take them for the
rest of their lives.
"These drugs are still relatively new and problems sometimes take years to show up," says Ott of the University of Washington.
"We’re
not quite sure what we’re dealing with over the long haul. Side effects
like this should make ordinary, healthy women think twice."
Piervin
still takes calcium and Miacalcin, a nasal spray that helps preserve
bone density but isn’t nearly as potent as the bisphosphonates. She
also walks every day and does weight-bearing exercise three times
weekly to help her bones stay stronger — even parks her car eight to 10
blocks from work to fit more walking into her schedule.
She’d take hormones, but she’s worried about the risk. She’d exercise more, but she doesn’t have the time.
"I’m off Fosamax," she says, "but I’m in limbo regarding future treatment."