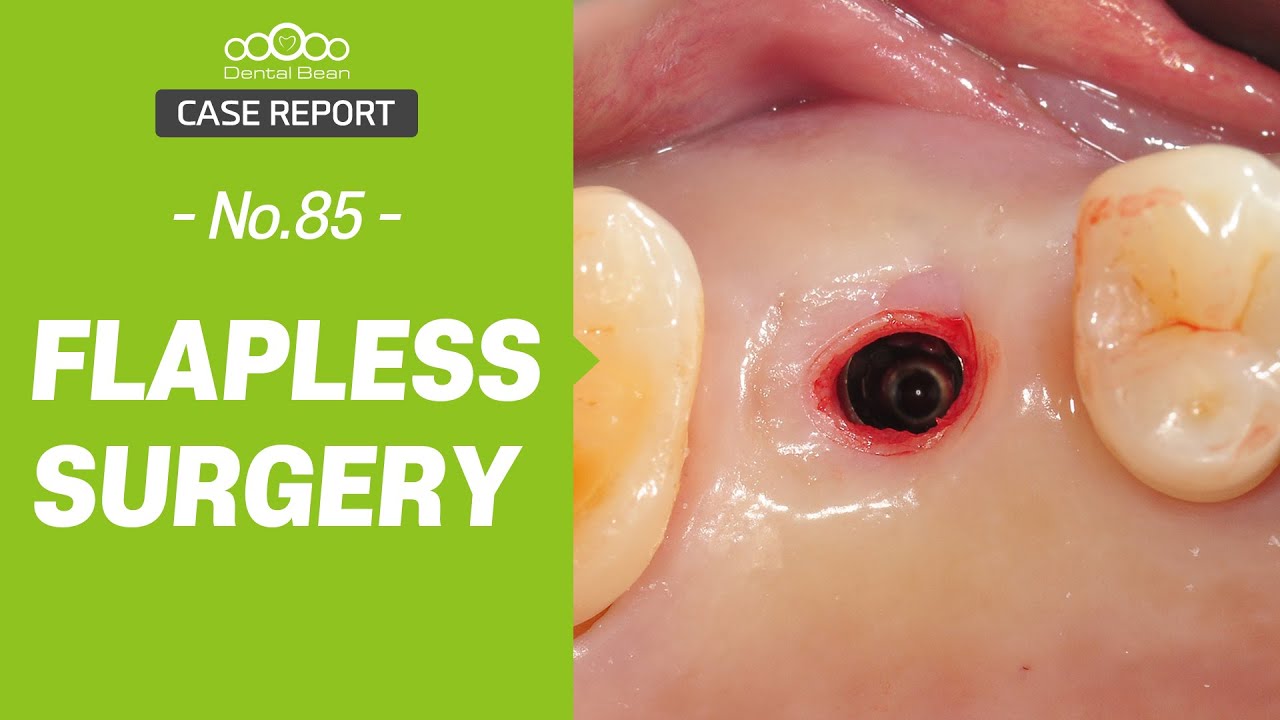
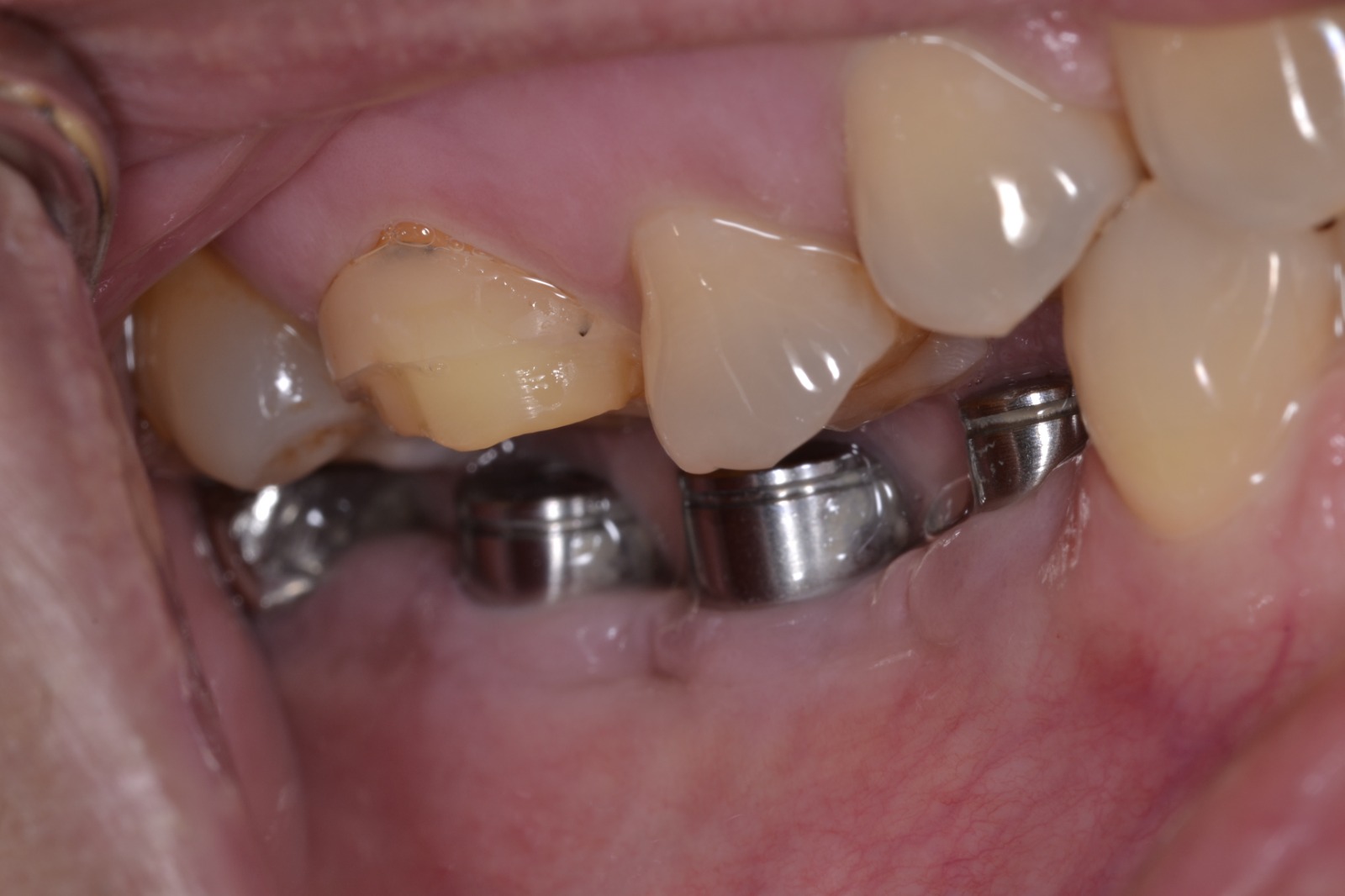
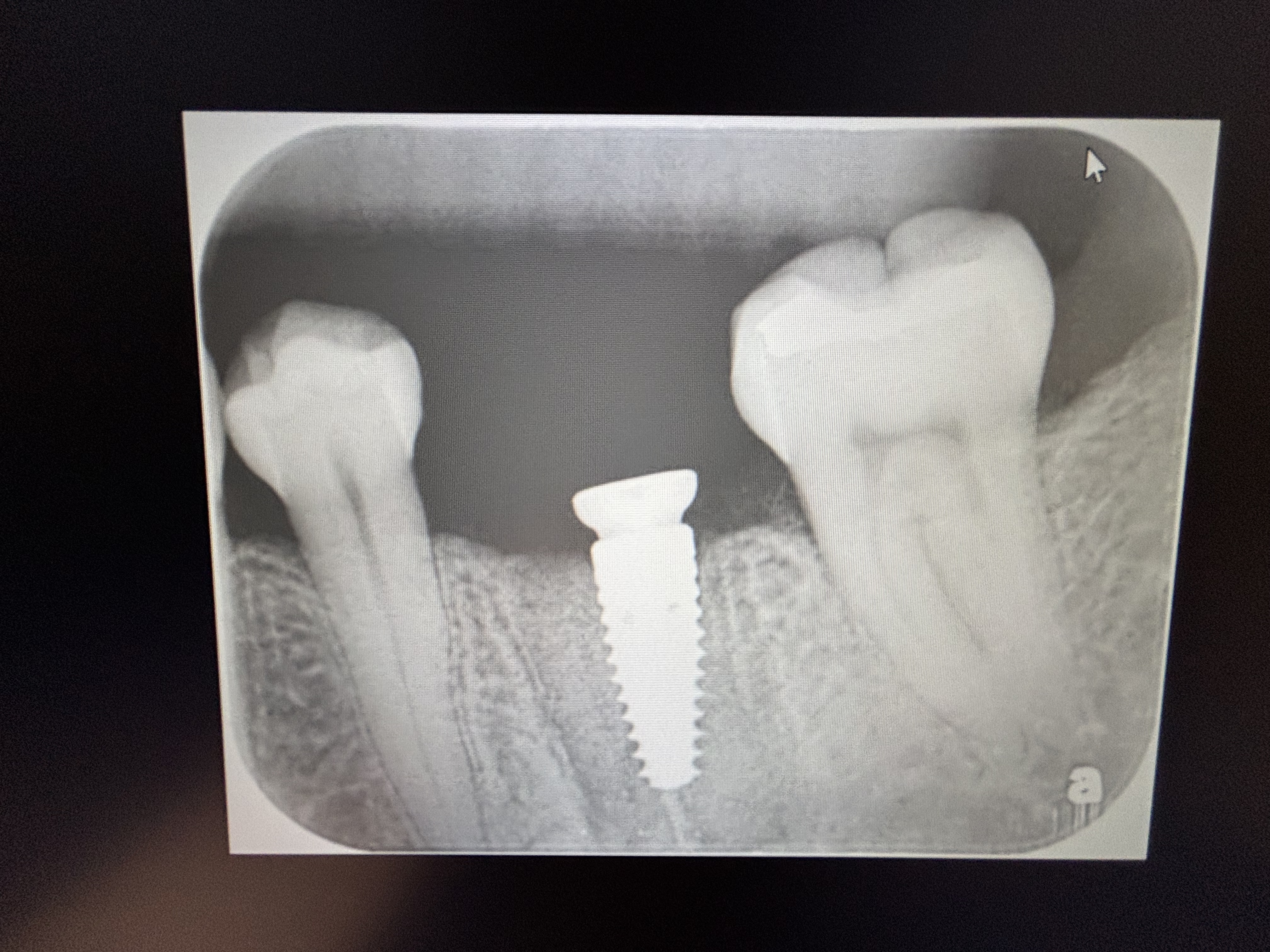
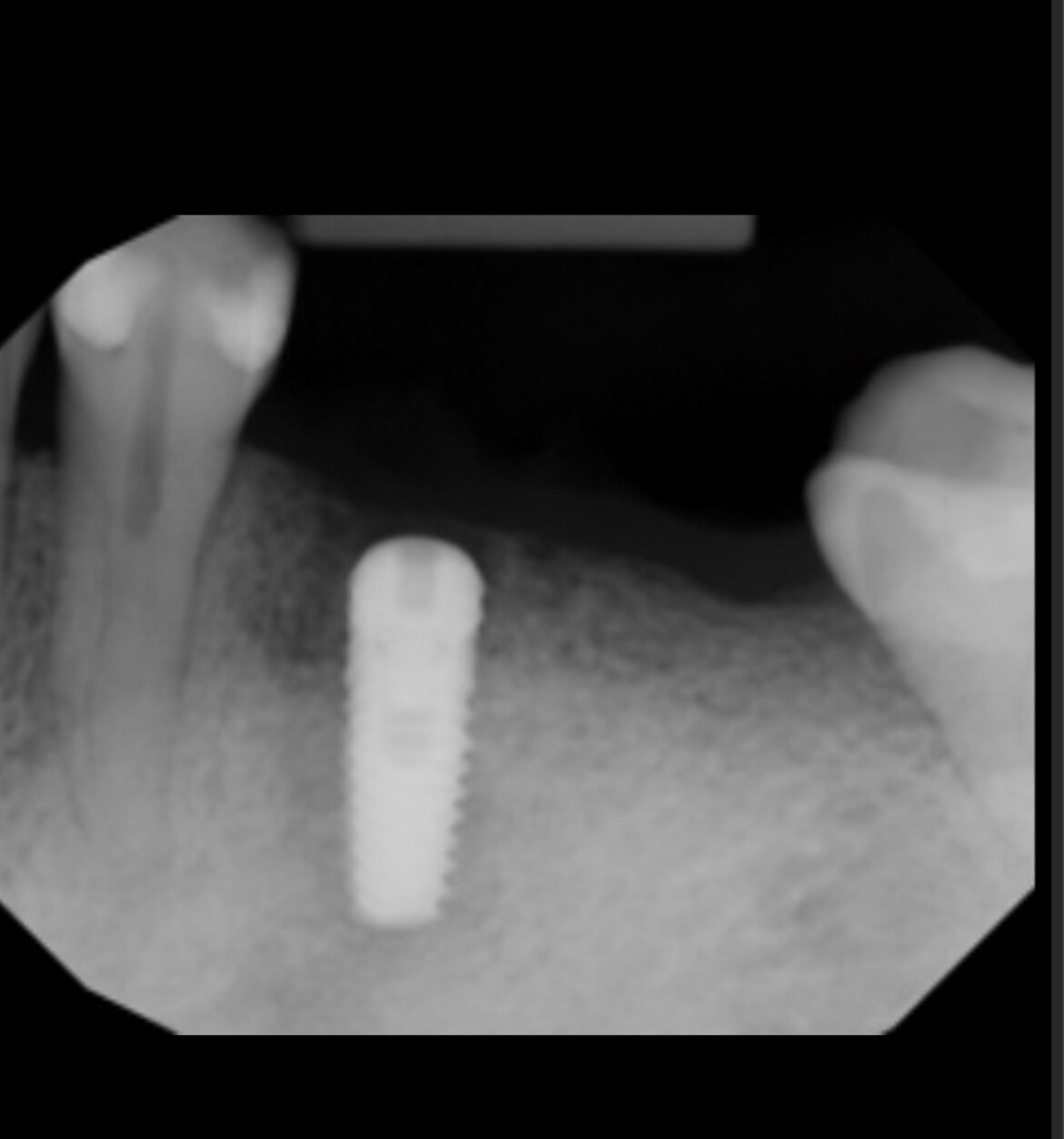
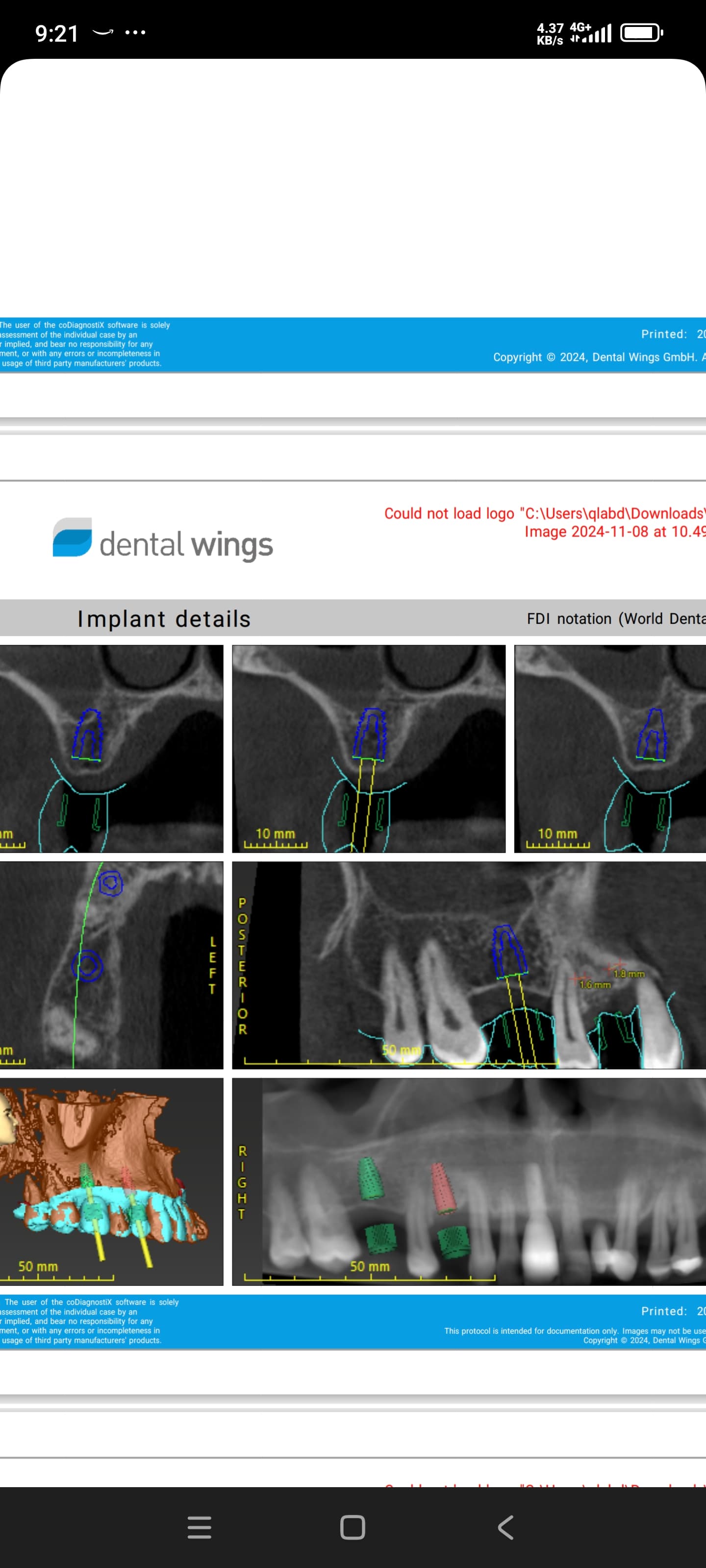
Nobel Direct Implant Reviewed
On February 6, 2006, participants from Medical Products Agency, Nobel Biocare and
clinicians met for a discussion regarding the amount of bone resorption
for the Nobel Direct dental implant.
Nobel Biocare presented data from the multicenter prospective study. The results for the Nobel Direct implant are comparable to conventional implants. The Gothenburg group appreciates the significance of the information presented in the study that encompasses 12 month prospective data. A constructive discussion followed. Based on the data presented at the meeting the Medical Products Agency has found no reason to withdraw the dental implant from the market. Nobel Biocare will continue to carefully monitor the development of the prospective study and has an obligation to act swiftly if compromising data should emanate.
Visiting clinicians presented data from ongoing clinical studies. The data from the Gothenburg group differ significantly from the rest of the multicenter data which they are part of. The Medical Products Agency as well as Nobel Biocare appreciates the significance and importance of the work of the Gothenburg group. The Medical Products Agency therefore urges Nobel Biocare and the Gothenburg group to meet and carefully assess the underlying data in order to explain the differences observed.
Lennart Philipson, Ph.D.
Director Medical Devices
Medical Products Agency
Participants
For the Gothenburg group:
Dr. Mats Hellman
Dr. Lars Jonsson
Professor Lars Sennerby
Professor Tomas Albrektsson
For Nobel Biocare:
Dr. Jeppe Magnusson
Dr. Robert Gottlander
Dr. Lars Enbom
Dr. Torsten Siepenkothen
Professor Daniel van Steenberghe, external consulting expert
For the Medical Products Agency:
Professor Björn Klinge, external consulting expert
Mr. Mats Ohlson, MPA
Mr. Arne Kardell, MPA
Dr. Lennart Philipson, MPA
Source: http://www.mpa.se