Peter, sure I agree with you to an extent. Experience is everything and obviously there is a slight difference in all these synthetics that can produce different clinical results in practice. In theory, it's similar to the known effect of generic vs brands in pharmaceuticals. It is a well-known fact that patients will react differently to brands and generics, even if in theory they are the same thing chemically.
That being said, the reality is that all these synthetic grafts (and types of membranes) are approved in the US via 510-K due to the substantial equivalence guideline, so by definition all the products are from a clinical perspective/result exactly the same. If they were different they would not get approved, and the manufacturers would need to seek a more extensive approval process with real trials (i.e. controls, statistical significance), which they never do in dentistry.
I think in the end, clinicians need to use what they are comfortable with, and in their experience has worked. There are a vast array of materials that will work because the materials and science are all already well understood. Really it all depends on specific circumstances and the different techniques clinicians employ.
Here in the US for example, allograft is abundant and doctors have had successful experience using it, so I think it's pretty much a given that allograft will be preferred in the US over other materials. Over in Europe, I believe that allograft is not widely available or even permitted in many countries, so obviously alloplasts (synthetics) and xeongrafts will prove more popular. Since the success rate of implants (and failure) appears to be exactly the same on both continents, I'm led to conclude that all these materials will work fine under the right clinician. Probably best to mix some of the materials together, which many do in the US (e.g. Dentogen (calcium sulfate hemihydrate) + Allograft).
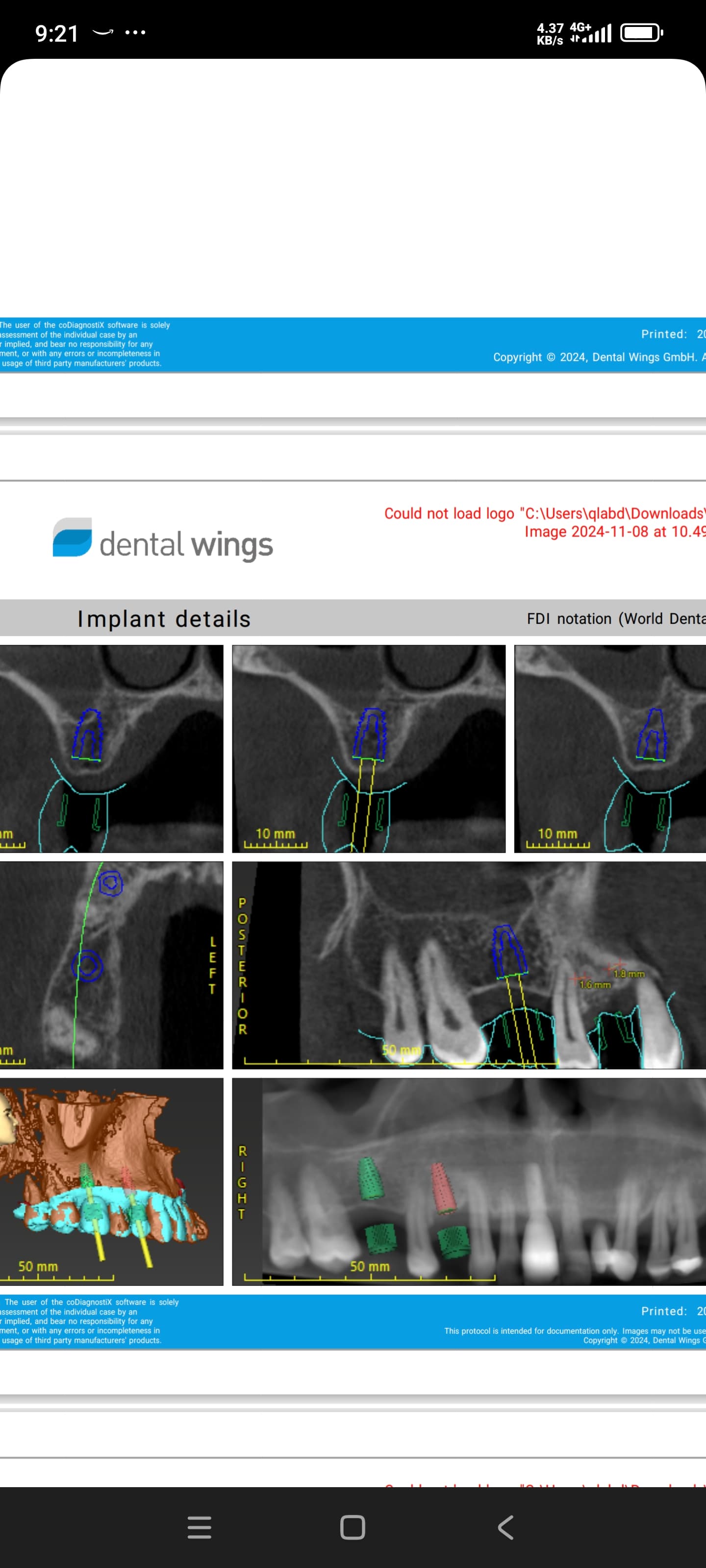
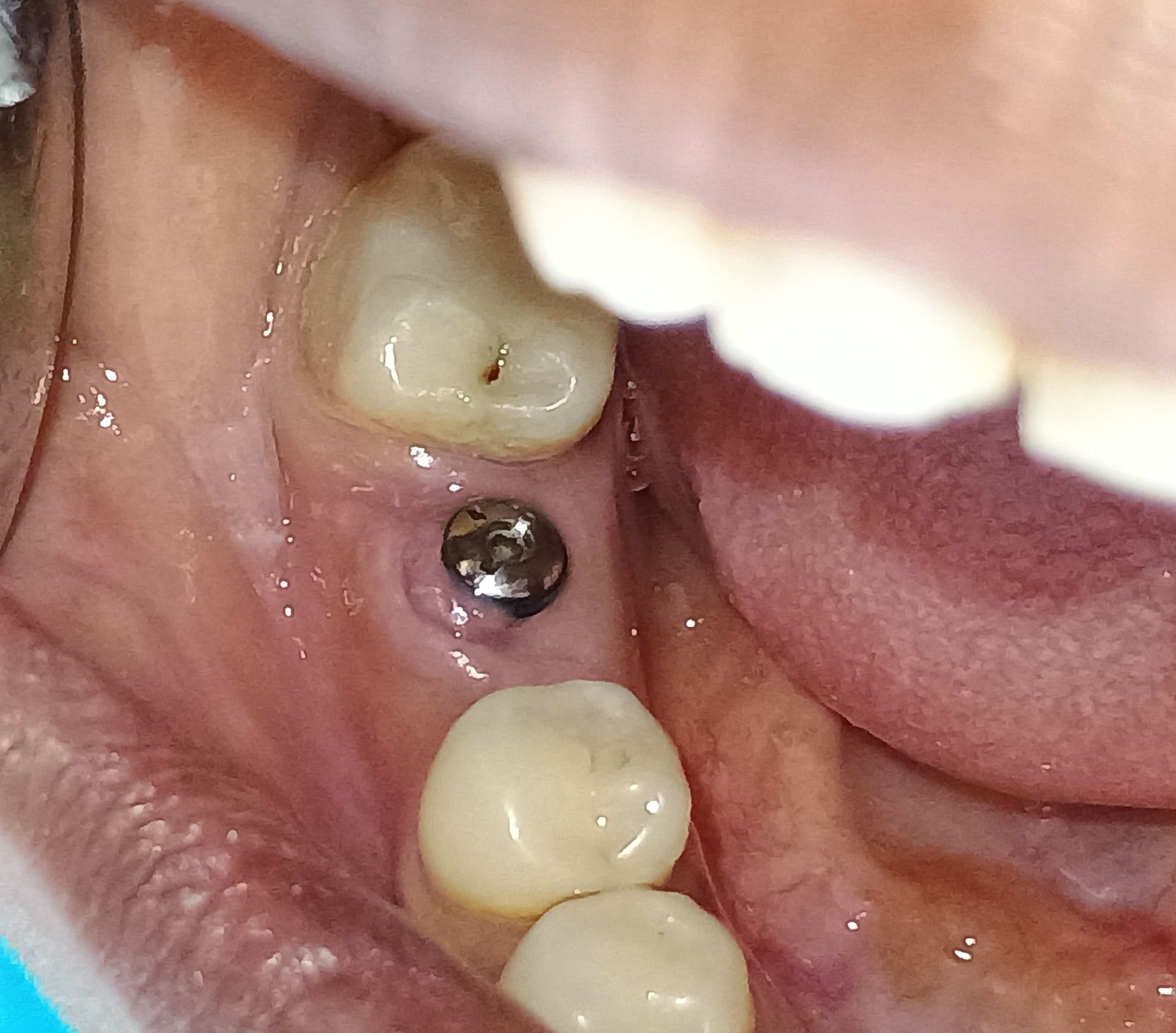
 There seems to be a difference in opinion on the need to cover a grafted socket with a membrane when there is no defect present. I would like to open the discussion up to the need (or not) to place a membrane in the following circumstances, as I find myself increasingly in this situation: A molar, upper or lower, is extracted and an immediately placed implant is planned. However, primary stability cannot be adequately achieved so the decision to graft the area is made. Or to take it a step further, an implant CAN be placed and subsequent grafting is done to “fill in the gaps”. What are people doing in this instance with regards to placing a membrane? I have read on this forum that people are not placing membranes and are having fine results with respect to grafts being retained and maturing nicely. Please share your experiences.
There seems to be a difference in opinion on the need to cover a grafted socket with a membrane when there is no defect present. I would like to open the discussion up to the need (or not) to place a membrane in the following circumstances, as I find myself increasingly in this situation: A molar, upper or lower, is extracted and an immediately placed implant is planned. However, primary stability cannot be adequately achieved so the decision to graft the area is made. Or to take it a step further, an implant CAN be placed and subsequent grafting is done to “fill in the gaps”. What are people doing in this instance with regards to placing a membrane? I have read on this forum that people are not placing membranes and are having fine results with respect to grafts being retained and maturing nicely. Please share your experiences.