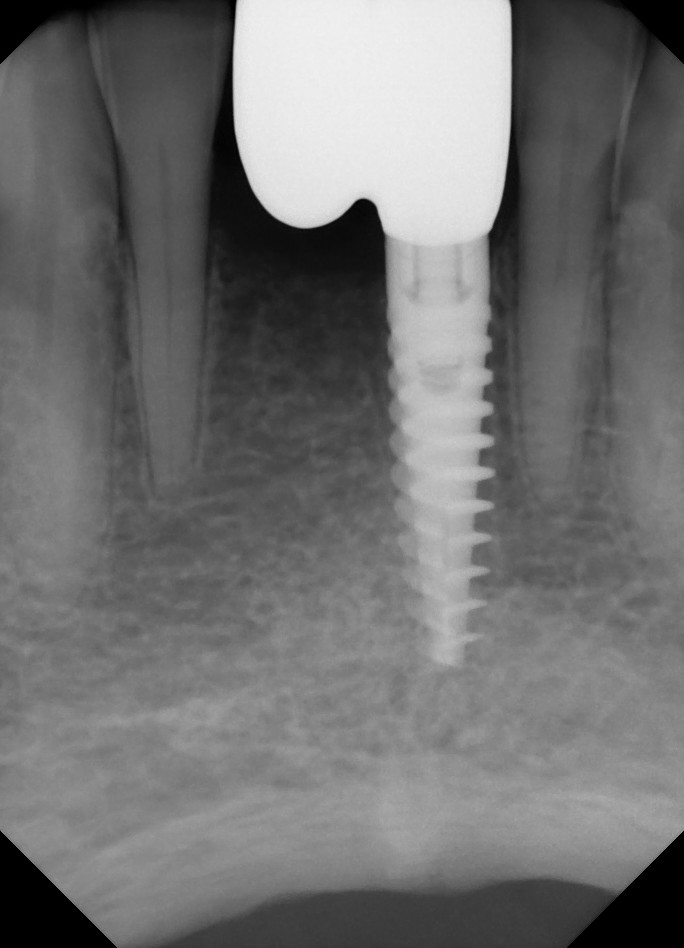
Using 3rd Party Custom Abutments?
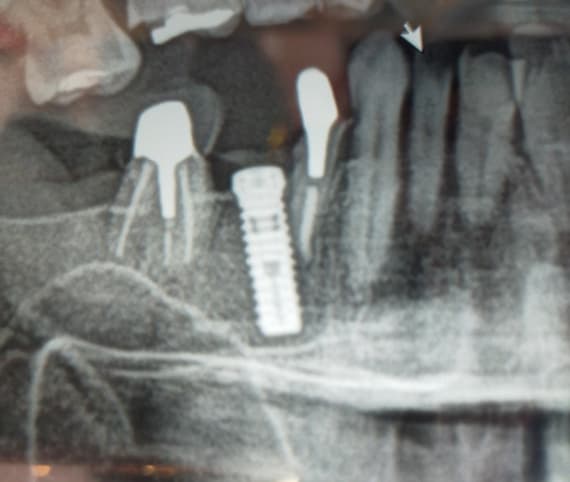
Here’s a case from yesterday. I’ve been using third party abutments for the past year due to PPO constraints, and they usually look fully seated at the implant level. But, after seeing this case, I think I need to take the leap to OEM parts only. The discrepancy at the abutment-implant junction has never appeared so vast to me, so I’m thinking it is due to non-OEM parts. Is this what you would possibly expect, if you’re using non-OEM parts?
As an aside, I checked my impression coping radiograph and it looks fully seated.

45 Comments on Using 3rd Party Custom Abutments?
New comments are currently closed for this post.
Peter Hunt
6/19/2019
Well it is good that you are checking in this way, most people would skip the radiograph at this stage and only find the problem when the final crown is placed.
The problems with most 3rd part connections is that the fit is less precise, i.e. the tolerances are greater. That makes them fit down readily enough, but it will increase the rotational discrepancy. That in turn increases the potential for wear and loosening of the connection. In short, the long term durability is compromised.
Another point to watch is that most implant manufacturers will not warranty an implant which has a third party component placed for a final connection. .
jwnicholsdds
6/19/2019
Hi Peter, thanks for your reply. Yes I always check to ensure abutment is fully seated. This time I was surprised because it never looks like this. I even tried to tighten it down again in case it wasn't fully seated but it arrived at the same position and I don't see any bone interference.
Is what you see here what you'd possibly expect to see from a third party abutment - that lack of intimate fit at the abutment/implant junction??
Gallo
6/19/2019
True on warranty but Atlantis does warranty their abutments even when the manufacturers don’t they will step up and cover the implant.
Joseph Kim, DDS, JD
6/19/2019
It is hard to tell what the problem is from the radiograph alone. What the abutment loose? Was the anti-rotational hex/octa/etc fully seated into the implant in the radiograph? Did you change abutment vendors? Are you sure the parts are completely compatible?
Any precision or lack of it when using 3rd party abutments or other components depends on the manufacturer of the 3rd part parts. I've been using Diamodent parts for many years, and lately make many of my outsourced abutments with TruAbutment. There are various reasons to use 3rd party components, including innovation (such as Zest Anchors), functional requirements (titanium alloy for thin abutments, or cast-to abutments), CAD/CAM not available through original manufacturer, cost, etc.
I just saw this article in the most recent edition of Implant Dentistry which concluded there were no clinically relevant differences among several of the components included in their study. Compatibility of Nonoriginal Abutments With Implants Evaluation of Microgap at the Implant–Abutment Interface, With Original and Nonoriginal Abutments. Duraisamy, Revathi, MDS*; Krishnan, Chitra Shankar, MDS†; Ramasubramanian, Hariharan, MDS‡; Sampathkumar, Jayakrishnakumar, MDS†; Mariappan, Saravanakumar, MDS†; Navarasampatti Sivaprakasam, Azhagarasan, MDS§. Implant Dentistry: June 2019 - Volume 28 - Issue 3 - p 289–295
Mwjddds, ms
6/19/2019
It's funny you say there was no clinical relevance amongst the fit of after market parts. In an article from the IJOMI (not sure exactly the issue) they compared Nobel stock abutments with Nobel OEM milled, Atlantis, argen and implant direct. All were less precise than the stock abutment for the conical connection with the implant direct abutment having the poorest fit. In this day and age where we should not be using flat to flat implant connections, but using platform switched conical interfaces, the precise mating of abutment to implant is critical for stability of the screw joint. Saving a few bucks on an after market abutment doesn't seem prudent vs. the cost of failure
Joseph Kim, DDS, JD
6/19/2019
I challenge you to show me an in vivo study, or even better, a systematic review on this topic that draws the clinical conclusions you are inferring. Also, how many of the pro-manufacturer studies are truly free of bias issues?
While I have no problem using manufacturer prefabricated components, 99% of my work involves custom CAD/CAM hybrid and titanium abutment work, or custom milled meso- and superstructures, which no manufacturer currently makes.
Gallo
6/19/2019
Have you ever tried Atlantis? I mwjdds it’s extremely important as I’ve had issues. Better to pay the price than to save pennies for issues that will come down the road.
Ed Dergosits DDS
6/21/2019
I am hesitant to say but my experience with Atlantis abutments has not been favorable. I had only one custom abutment fabricated for one of Dentsply's implants. The I/A "fit "was like socks on a rooster. The fee was ridiculous.
Joseph Kim, DDS, JD
6/21/2019
Yes, I've used Atlantis in the past. At the time, they were technically at the top of the industry. They are still an excellent company, and if I didn't have my in-house laboratory, I would definitely considering using them.
Greg Kammeyer, DDS, MS, D
6/19/2019
I recently sat down with my Nobel Biocare district manager. He showed me case after case of fractured implants (flowered open) that had been restored with abutments made by a local laboratory. I'm sure many of the abutments work out fine yet I wonder how much R & D goes into making sure that no matter what implant brand an abutment goes on, that the parts fit as well as the "Name Brand". Since I prefer to pay full fee for my dentistry, I want what is truly "best". I believe our patients THINK that is what we give them, yet there are countless ways to decrease overhead and or patient fees. For a dental implant, this savings can cause significant problems: all the implants he showed had to be removed. I believe if you have serious financial constraints, it would be better to use a clone implant with their original parts than to accept the misfit of mix and match hardware.
Ed Dergosits DDS
6/21/2019
I totally agree Greg. If one chooses to use an extremely over priced implant Like Nobel they should follow up and restore the case with an extremely overpriced abutment made by Nobel. A dentist can also choose to have the crown made by Nobel at an extremely overpriced lab fee . A dentist has the choice to charge a patient an extremely overprice fee for the procedure so he or she still makes a profit. This is often true. I often have patients that seek a second opinion that is often financilly driven. Today I had a patient that was quoted a fee of 3k for implant surgery without a graft, 800 for a custom abutment and 2k for an implant supported crown. This fee was supported by the use of "swedish" engineering. I was basically in shock. If there are other dentists out there that think these fees are reasonable I am further in shock. You get what you pay for? Oh please!!
Joseph Kim, DDS, JD
6/21/2019
Depending on the region of the country, the fees you are quoting may or may not be normal. Having said that, I have encountered patients who have presented for a second opinion that have produced some outrageous estimates relative to the complexity, or lack thereof, of their particular situation. As the case size and complexity increase, my billing practice is to consider how much time and risk are involved when determining the final fee. For example, placing and restoring seven implants in four quadrants, including the esthtetic zone, will cost more than placing and restoring seven implants to rehabilitate an edentulous arch that require no significant grafting. At the end of the day, my fees must match my community's ability to afford the treatment, which is solidly middle class in my area. This means I have to find ways to perform the procedures with greater predictability, less chair time, and minimize the prosthetic costs.
I routinely have patients drive out from or near Chicago, who have been referred from colleagues or other patients, wanting to save tens of thousands of dollars on their treatment. While I do not advertise my fees, it seems to me that price definitely matters.
Joseph Kim, DDS, JD
6/19/2019
I'm not surprised to hear of Nobel's fractured implants. From the pressure osteonecrosis scare, to the flowered narrow diameter trilobe implants, to the suppression of Nobel Direct and original all-on-4 disasters and subsequent departure of Albrektsson, Nobel has many skeletons in their closet. Mechanically speaking, I am unaware of any study claiming 3rd party abutments causing increased fracture to Nobel implants. What we are left with is a collection of terrifying pictures that will help keep the proverbial sheep in the fold. Aside from the manager's (self-interested) claim, how do you know the abutments weren't actually Nobel's? What was the status of the bone around the implants at the time of fracture? How biomechanically sound were the prostheses of these failed implants?
Over the years, I learned 1) sales people and their managers will say anything to sell you something, and 2) we should never forget that we are the ones with the doctorate.
Robert Teague
6/19/2019
Not all sales people say anything for a sale. Sorry, I feel a need to defend the professionals amongst the group. In such a generic statement. Otherwise I agree with your commercial points. I would hope Nobel check the images provided to their reps to ensure such claims/statements are true and correct.
Keith Goldstein
6/19/2019
The other issue is many of the 3rd party manufacturers of custom abutments and ti bases have not gone through FDA approval on the products they are selling so there is no 3rd party validation of the tolerance or fit or compatibility of the components and screws that they manufacturer. Plus the quality control with parts measurements is severely lacking where they do not use proper metrology testing devices on the parts that are made consistently. Also you, as the dentist, need to check with your lab and milling centers if they have FDA approval on that EXACT part and screw and if it is not stated in that document then do not use it. There are no substitutes.
Neil Park
6/19/2019
FDA does not approve the fit of implant parts. The products are cleared through a 510(k), which does not examine fit. I think the crux of the issue that is being discussed here is related to the lab's protocol for milling the abutment. The approved way is to use an abutment blank, which already has the implant interfaced milled onto it. The lab is then milling only the "prep" part of the abutment. Some labs are milling the implant interface as well, which will never result in the same type of fit as a premachined connection.
Timothy C Carter
6/19/2019
As a general rule 3rd party components are a no go. If you are using a name brand fixture it will have a lifetime warranty as long as genuine parts/pieces are used for the restoration. The parts are not manufactured to the same standards and I can assure you that prosthetic issues will arise if this practice continues. I will say, however, if you are trying to keep overhead down Blue Sky Bio makes the best discounted implants and prosthetics I have seen. I currently use all genuine Zimmer implants and components as I am sold on their quality but if I were to be forced to use a lower cost product it would be BSB (I have placed a lot of BSB in the past and have not had any issues).
Joseph Kim, DDS, JD
6/19/2019
You make some bold claims, but this one especially caught my attention, "The parts are not manufactured to the same standards and I can assure you that prosthetic issues will arise if this practice continues." Do you have any references or citations to back this up? I'm not aware of any reputable manufacturer that has these prosthetic issues that you claim. The irony of your statement is evident by your support of genuine Zimmer parts, and compounded by your mention of BSB.
The former owner of Corevent/Paragon, Gerald Niznick, sold his implant company to Zimmer, only to run his machines at Implant Direct literally the minute after his own patents expired. Wouldn't you agree that the inventor of the Zimmer internal hex connection would know how to manufacture the same or better? Through Implant Direct, Dr. Niznick proved that 3rd party components that were the exact replica of the original, were equivalent to OEM. The ultimate proof was when Danaher, which owns Nobel, bought Implant Direct to shut him up.
The warranty manufacturers offer is subsidized by the huge markup they place on their products. For example, the raw materials to manufacture an implant may be about $0.25, with a little extra for some of the surface preparation and sterilization steps that are usually outsourced to another vendor. Stock abutments don't require surface preparation nor do they need to be sterilized, so the production cost of these is likely less than the implant. Yet, many manufacturers will charge $150 for a stock abutment, using a small portion of the markup to cover their warranty. In my opinion, unless you have a very high failure rate using manufacturer components, the economics just don't justify their prices.
Timothy C Carter
6/19/2019
You completely misinterpreted my comment. I have seen mixed/matched products produce prosthetic complications that I have not encountered when using genuine Zimmer. I said that I think BSB makes good stuff and if I were looking to cut cost I would recommend BSB implants and parts. This is just based on my personal observations over the past 15 years
Timothy C Carter
6/20/2019
I said I use genuine Zimmer (my preference). I believe the same could be said for genuine parts from 3i, Straumann, Astra, etc... Just use parts designed for the specific implant. I drive a Ram 2500, again my preference, I know Ford and Chevy make good products but I prefer the Ram.
Joseph Kim, DDS, JD
6/21/2019
I have no problem with clinicians who choose to use original manufacturer components to restore their implants. However, clinically acceptable results can be achieved by using 3rd party components, especially for the Zimmer platform. I agree with you that prosthetic complications can arise from mixed components, but you will often find that the clinician behind the failure either didn't inspect the fit of mixed components prior to using them on their patient (preferably under 20x or greater magnification), or is the type of person who has no problem putting profit ahead of patient. I have seen many poorly manufactured 3rd party components for various platforms, but that doesn't mean that all of them are horrible. In fact, many of them produce parts that are mechanically equivalent to those from the original manufacturer, and some who offer additional features that are unavailable from the original manufacturer. In this thread, I have mentioned Zest Anchors multiple times as a third party manufacturer who provides excellent components that are not even available from the manufacturer. I don't hear anyone complaining about poor fit or function regarding the connection being milled by them. Similarly, there are CAD/CAM abutment manufacturers who also produce excellent products, such as TruAbutment and Atlantis, among others.
Joseph Kim, DDS, JD
6/19/2019
FDA approval does not indicate "3rd party validation of the tolerance or fit or compatibility" of anything. It merely indicates that the FDA approved item can be marketed to accomplish the claim it is making. Having said that, it is desirable to use FDA approved products when available, although clinicians are able to use products in an off label fashion as needed.
Perhaps having my own in-house lab leave me biased, but I still remember waxing and casting full gold UCLA abutments and later cast-to abutments, neither of which fall under FDA jurisdiction, even though the former was barely acceptable relative to modern standards. Apart from a published independent evaluation of a particular product, reputation and customer service have become the most important factors in my daily practice.
docphil
6/19/2019
Great points. Technically, though, as mentioned above by Neil, the FDA doesn't approve any of these items. The correct term is really FDA "cleared", as they are cleared for sales via a 510-K showing "substantial equivalence." 510-K's are not complicated. Furthermore, even when there is FDA approval (Class III), it doesn't necessarily mean the FDA validated anything other than some written studies. With so much of manufacturing done overseas now, particularly in China, the FDA is severely stretched and doesn't even visit most manufacturing facilities. There is a massive quality control issue, particularly as it relates to pharmaceuticals, let alone medical devices, like implant abutments. Highly recommend reading: "Bottle of Lies: The Inside Story of the Generic Drug Boom" to get a sense of the craziness that is going on now with FDA and overseas manufacturing, FDA jurisdiction etc.
Gallo
6/19/2019
Let’s get started omething straight....FDA does not approve its 510/cleared for sale and use. Would you rather have a biometric knee implant or have the surgeon be cheap and use some knock off that piggy backed on the original FDA submission and made it cheaper in Taiwan?
CPKW
6/19/2019
I wonder how many dentists trust Non-OEM parts on their own cars......just because they are cheaper ?- or would they trust original parts more- just a thought ….
Joseph Kim, DDS, JD
6/19/2019
The entire aftermarket car industry exists solely because a part that is needed or desired is not made by OEM. In the dental industry, 3rd party ti-base connections for screw retained restorations are often superior to OEM. There was a time when Atlantis custom abutments were the only CAD/CAM abutments available. The Locator abutment and Dalbo system also come to mind.
This thread has really surprised me in regards to the level of information, or rather belief, that is present in this cross section of clinicians. There is nothing magical about what the original manufacturer is doing that cannot be duplicated by another, unless prohibited by intellectual property restrictions. It's not like the original manufacturer made the CNC machines, nor owns the employees that tend to jump from company to company. Nor do they own the titanium mines and refiners or the stock rod producers. Short of tool life issues, there really is little to no differences between manufacturers who are using the exact same equipment and the exact same digital files to produce their products.
jwnicholsdds
6/24/2019
Joseph,
Thanks for your comments and wisdom on this thread brother. This comment in particular is intriguing, because I was under the impression that the makers of OEM parts had some sort of proprietary information about the intaglio of their implant, which other companies are unable to replicate, therefore creating a mismatch. You seem to be refuting this. If what you're saying is true, why aren't third-party parts universally understood to be analogous to OEM?
Joseph Kim, DDS, JD
6/24/2019
Even if a 3rd party company has their hands on the exact same data files to make the OEM part, the final product may deviate in the following ways: 1) milling strategy, or the instructions from the computer to the milling machine, may be different, 2) tools may be different or overused before changing, 3) finishing steps may be different. Thus, exact reproduction may or may not be present. This is why I mentioned that reputation and personal inspection under high magnification is necessary to ensure our patients are receiving equivalent products.
Priyu
6/19/2019
501(k) is FDA Approval and does not prove precise fit. In other words, even if the manufacturer has a 501 (k) approval, doesn’t mean it has the fit of original manufacturers parts. These connections are patented and are not duplicated to the exact parameters which is exactly what we need to properly restore an implant. And it is evident in the X-ray that this abutment’s design does not seat exactly on the implant. I would stick to the same manufacturer for all restorative parts.
docphil
6/19/2019
510K is not FDA approval. It's clearance for Class II devices based on substantial equivalence. FDA Approval is only for Class III devices. 510K is FDA Cleared. What is the Difference Between Cleared and Approved?
CPKW
6/19/2019
there is no evidence that third party components are "superior" to OEM, many have patents protection. even the screw head and thread designs are not often compatible and can be very different. The main reason third party components are made is because they are cheaper- nothing more. There is no guarantee of quality control or manufacturer variation- why would there be. Common sense alone suggests that in general OEM should provide better function and the literature does support this. treat others as you would wish to be treated.
Joseph Kim, DDS, JD
6/19/2019
An OEM ti-base that is 3 mm in height is not superior to a 3rd party ti-base that is 5 mm in height in most mechanical circumstances. Also, a nonexistent OEM part is not superior to an existent 3rd party part, such as the ones I reference above, Locator, Dalbo, and many others.
mark
6/19/2019
Any time you are going to cut corners you have to expect the faulty fit to land on you. If you find it necessary to do this, I have to wonder why. You may save a couple dollars but it would be better just to order the standard of care abutments and charge the patients a fair fee. If you find yourself forced to use these discounted attachments at least do the following. See if they are FDA registered. It will have a patent number which is easy to check. If you are like me and use one main implant system, always try the fixture on one of your implant platforms to verify it is fitting properly in the internal or external hex and yes, take a digital bench X-ray and look at it under high magnification.
Dr Dale Gerke, BDS, BScDe
6/19/2019
This is a fascinating discussion and one the dental profession needs to fully consider.
About 5-10 years ago I attended a seminar (cannot remember which brand) where the claim was made that failure rates of genuine components was about 3.5% and double with non genuine parts. The presenter (a dentist but obviously paid by the brand) then proceeded to do some calculations showing that by using genuine parts, you actually saved money. I was left unconvinced.
Clearly with CADCAM developments over the last 10 years (particularly scanning and milling accuracy) it is possible to properly generate a non genuine part. However as with any third party involvement, it is critical for the dentist to check accuracy and quality before using the finished product. We have all experienced unsatisfactory laboratory work and, for those who care, decided to only use competent laboratories in the future (which are usually justifiably more expensive). The problem for us with implant components is judging the quality. Strength and accuracy are difficult for clinicians to judge because visibility is poor and some tests require engineering equipment.
Here is my concern which became apparent to me from bitter experience.
Genuine parts are usually much more expensive than non genuine (probably at least double but possibly up to 4 times more). But in reality the cost to the brand company is around $5 or less (the part is usually only a bit of milled non precious metal). Of course the brands all tell us how much the R&D has been etc. But truthfully the required cost of development has been paid and the brands are only getting “add on value”. Then of course the brands all maintain that they will only warrantee the implant if the components are genuine. Added to this is the clinical dilemma of medico-legal issues (does using non genuine parts lessen any malpractice defence).
However when there is a failure of the genuine component, the brands only provide replacement parts – they do not pay for the laboratory costs or the clinicians’ time. I can live with this when the problem is an implant failure, because who is to know which entity caused or contributed to the failure (in truth it is most likely to be the clinician). However when it is a prosthesis failure (eg abutment) then I feel that replacement should be fully paid for by the implant company.
To explain my position more; about 10 years ago I was using a genuine cast gold abutment system which was promoted by the implant company to be used in bruxing situations. I liked this system philosophy because I feel there are many good reasons to use porcelain bonded to gold abutments to avoid any potential crevices or cement irritation at the crown-porcelain-abutment margin. Unfortunately the system failed in four cases after 1-3 years due to casting distortions in the abutment-screw channel which lead to differential stress on the screw and subsequent screw fracture at the abutment-implant level.
Since this was obviously a component failure issue, I asked the brand to pay the laboratory fees and my costs (I had to remake all crowns). After much legal argument, the brand reluctantly settled for a lesser amount on three cases and the last one is still pending (although an unacceptable offer has been made). I have since changed brands after approaching several reputable companies and requesting a letter stating that if there was any component failure, they would pay full cost of any replacement prosthesis. One brand conceded my history was good (no previous failures) and my request was reasonable and they provided me with such a letter. I switched to them.
My point here is that the brand warrantee issue is in reality a non event. Inevitably if failure happens, the brands will replace the part with a component which in truth costs them very little, but we have to pay the laboratory costs and also our time and expenses. So actually, using cheaper non genuine parts (which fit properly) is actually much more economically sound. Thankfully the brand I now use has allowed me to confidently use genuine parts because they will pay all replacement costs if required (to this date, this has never happened).
I would encourage all the profession to reflect on this question. Would we accept a “new car warrantee” which (in the event of a serious problem – eg an engine or gear box failure) only provided a replacement part but all other costs of labour etc would need to be paid by us - the consumer? I do not think any of us would accept such a warrantee and neither would most consumer protection authorities.
So why do we as a profession allow the implant brands to provide us with a most unacceptable warrantee on parts? Probably because implants are relatively new and there have been many variables involved with failures to date. I understand this and accept it from an historical perspective. But implantology has advanced considerably and we and our patients deserve better protection for component failures (it is not as though the implant brands have such narrow margins they cannot afford it).
I would be interested if others feel it is time for all of the profession to be demanding more in this regard.
Dr. Gerald Rudick
6/19/2019
Judging by the number of dentists who have responded to this discussion, it is obviously a topic on the mind of a lot of people. Each time we deliver a screw retained crown, it is a " a third party custom abutment" and not an original abutment manufactured by the implant company that has made the actual implant.
The idea of an internally hexed implant was actually the brainchild of Leonard Linkow, who took this idea from the furniture industry and applied it to implant dentistry, when the furniture manufacturer supplied an adjustable foot for the leg of a table...….it is an internal hex fixture, that Gerald Niznick ran to the patent offices aroud the world and held the dental implant manufacturers hostage until his patents ran out after twenty years......but the point is....we have not found a perfect or even satisfactory design to place a crown or abutment on an implant...… the engineering of having a huge component ( crown) that takes a tremendous load and is secured by a tiny hex to restrict twisting or turning.... it is bad engineering, and we must find a better design....the one piece implant that combines the implant and abutment is a better solution to the problem, but presents problems of its own, if we want to ensure that the implant is not loaded during the process of completing osseointegration.....so all you geniuses out there, put on your thinking caps and come out with a better design, so that we will be rid of screw loosening, and fractured implants
Ed Dergosits DDS
6/21/2019
Gerald The platform switched internal connection basically solved every I/A connection "issue" almost 10 years ago. Anyone using a flat connection without platform switching is basically practicing in the dark ages.
Mwjdds
6/19/2019
Amazing discussion! Having restored implants for 30+ years, and having seen the engineering changes over the years, here's my thoughts.
In the early days, abutment fit wasn't a big deal. We had flat implant heads with either an external or internal connection, neither of which were great. So, we torqued the abutment screws to 30-35Ncm to clamp the parts together. Poorly fitting aftermarket parts were ok since it was all about torquing the screw. No we have precisely engineered implants and abutments machined to work together without needing to torque the heck out of an abutment screw to keep the parts together. The internal cone of the implant was machined to match the precise cone of the abutment so we don't have to torque to 35Ncm anymore for a stable joint. However, when labs " save you money" by using less precise aftermarket products you run the risk of less precise fits which increase forces to the lateral walls of the implant and to the screw. Also, aftermarket parts have different screw head sizes and screw head seats so, if an abutment screw fractures, you need to know who manufactured the abutment so you can get the correct replacement screw. My point is.... document the abutment used for future reference just like you document your implant system. Use quality parts. What would you use on yourself or a family member? What's the cost of failure? Is it worth the few bucks you save by using aftermarket parts?
We are implanting biomedical devices in human beings. When did we decide the patient doesn't matter? That it's all about cost?
jwnicholsdds
6/24/2019
Thank you for your reply. You addressed something I asked my rep about recently but she wasn't sure...the abutment screw is specific to the abutment, not the implant?
Therefore, if you have a new patient to your office w/ a loose screw and need to order a new one, how are you supposed to know what screw to order if it matches the abutment which you don't know what type it is? I always thought it was related to the implant but now i'm not so sure.
Vasant Ramlaggan
6/19/2019
I just recently had to remove an old implant crown over a Nobel Groovy implant I first fitted about 6 years ago. At the time, my lab could only use a third part abutment (crown was cemented over it on a max first molar). Upon removal of the crown, I could not easily get the abutment off and had to resort to extra work to get the over-tightened fitting abutment off. That was hell! I don't want to go through that again! Any time I've used Nobel parts for my Cerec machine, I've come out doing well! I'm switching to another implant company for other reasons and will use THEIR parts, not a third party for my implant restorations!!!
Ed Dergosits DDS
6/21/2019
Vasant what was the reason that the crown needed to be removed from the abutment? Why would you think this was an issue with custom abutments made for Nobel Groovy implants? Your report lacks information that would make it make sense. Please explain.
Vasant Ramlaggan
6/21/2019
Hi,
My patient is a severe bruxer, has apnea, reduced vertical, etc. The crown loosened up (probably due to the parafunctional occlusion) and this, as I mentioned, was before I started doing more screw retained for posteriors. The issue I had was removing the third party abutment from the implant itself: It binded too tightly and I had to cut the inside of the metal part of the "ceramic" abutment which would not come out of the implant. I was able to cut the abutment base internally enough to alter the abutment shape and squeeze and loosen it up.
I have very successfully removed abutments under cemented crowns in the past and this was the first time I had this type of issue.
Ray Kimsey
6/20/2019
Lots of disagreements in these posts but I do know that my lab once used a non-manufacturer milling lab for a custom abutment and after 18 months the milled abutment had corroded and fractured. Never again.
Peter Hunt
6/21/2019
May I be allowed to come back into this discussion with comments about the stability and strength of implant connections. The integrity of these joints basically comes down to a few very simple principles.
First, the deeper the post the more stable the joint and the more the forces coming down on the crown are dissipated throughout the whole implant. It’s much safer to have a post with depth greater then 5.0mm than one that is only 2.0mm deep
Second, it helps if the anti-rotation components have minimal rotational discrepancy. They can range considerably from about 1.5 to as much as 7.2 degrees. This is important as components go in and out of the mouth several times during the restoration cycle and back and forth to the lab. This can screw-up contacts and if the case is splinted it may not seat fully.
Six or eight potential positions are very common. The trouble is that these hex or octa positions contact at an angle and this inherently increases the rotational discrepancy. If the anti-rotation component is set at 90 degrees to the rotation path, the rotational discrepancy decreases considerably and there are less “explosive” forces act to “Flower” the implant.
Flat platform systems have one aspect where they are clearly superior to conical systems in that they have less vertical discrepancy. Flat platform systems come in at about 10µm discrepancy, conical connection systems start at about 20µm and can range up to more than 40µm. In addition these get worse, up to as much as 100µm, with repeated insertions as titanium debris is generated and gets packed into the joint.
So by very simple analysis of a connection, it’s possible to predict how it will perform in the long run. Deeper joints are better, if the anti-rotation component is set at 90 degrees it will have less rotational discrepancy and less likelihood of “Flowering” the implant, and restorative therapy will be simpler, more precise and more predictable.
jwnicholsdds
6/24/2019
Wow, I certainly wasn't expecting this type of response to my question. Thank you all for your wisdom and input. My major takeaways:
-We don't really have hard evidence to take a stance either way. As evidenced by the dispute above. That's probably why non-OEM continues to thrive. Based on the explanations above, my own clinical experience and ethics, I think going with OEM parts is just a safer way to go and what I would want in my own mouth. If a PPO fee doesn't allow for enough of a margin, a separate fee can be added and explained to the patient.
-I'm not willing to sacrifice on implant quality - there was a suggestion to go w/ a clone implant. So if that's the case, I just need to go with an OEM
My original question wasn't really addressed - whether the gap seen in the attached radiograph is likely attributed to non-OEM part vs. alternative, but I imagine that it is due to use of non-OEM. Praying now for no issues in the future.
Well, this is dentistry. All we can do is make corrections after mistakes are noticed, instead of repeating the mistake again and again!
tcarterdds
9/3/2019
I just had to repair another case in which an Implant Direct abutment was used to restore a Straumann Tissue Level fixture. Once again screw loosening when an ID abutment is used which strengthens my case that ID "compatible" parts are a problem waiting to happen.